|
SEPTEMBER: WEEK 4
September, 27th
MiniPrep and E-P digest for I62-a, I62-b, I62-c, I62-d, I62-e, I62-f
DNA pcr for MyYeast 1-2-3
Gel run
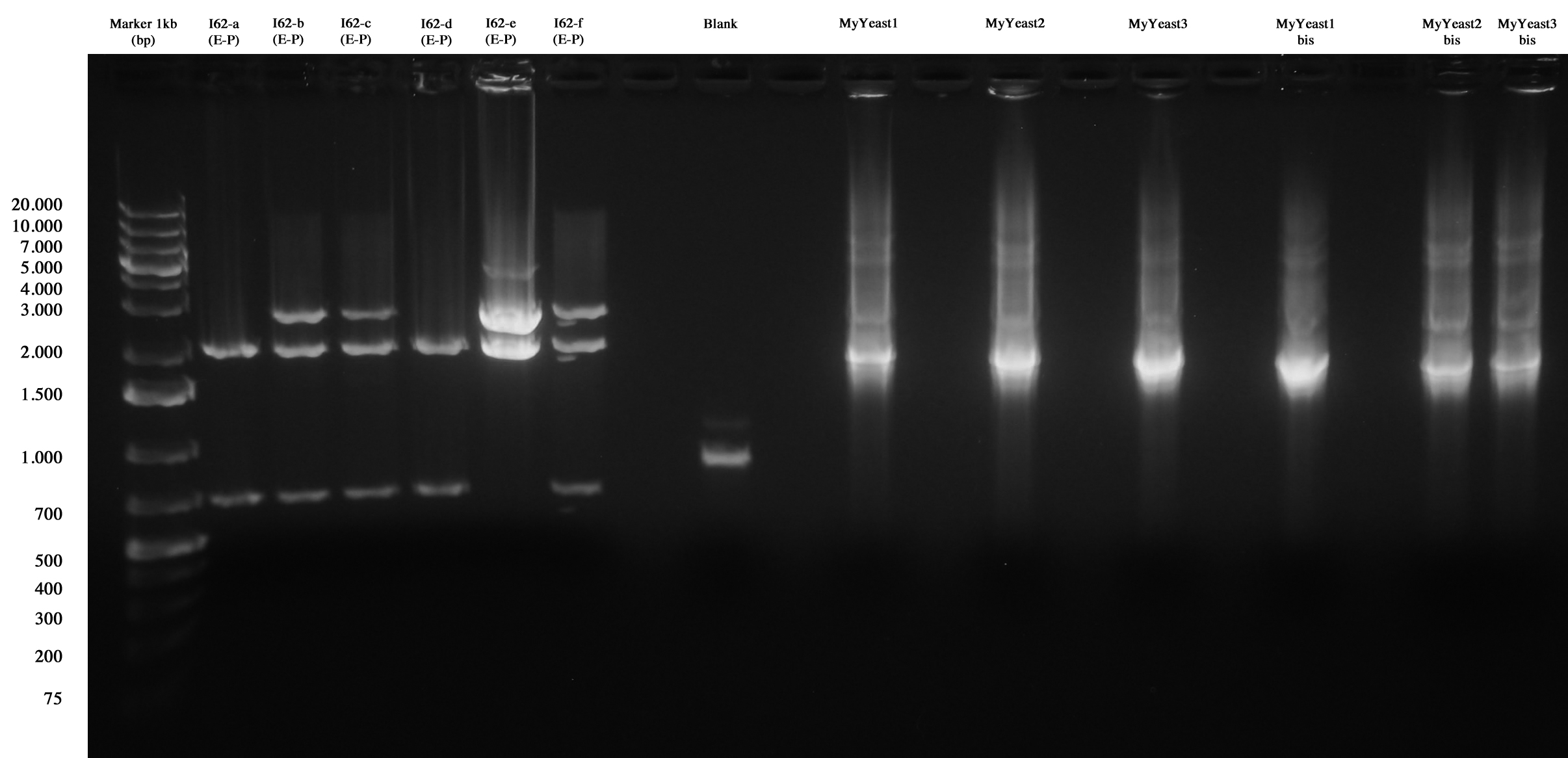 I62 and MyYeast series screening (I62, blank, MyYeastx,xbis).
I62-e was positive, so we made glycerol stock and stored it at -80°C.
Protein electrophoresis for phasins (Fede)
September, 28th
Tecan Test Giacomo
Miniprep and quantification for:
- I1: 59,6 ng/ul
- I4: 109,9 ng/ul
- I53: 160,3 ng/ul
- <partinfo>BBa_J04450</partinfo>-1:55,9 ng/ul
- <partinfo>BBa_J04450</partinfo>-2: 47 ng/ul
ON digestion of:
- I1 (E-S)
- I4 (E-S)
- I53 (E-X)
- <partinfo>BBa_J04450</partinfo>-1 (E-P)
- <partinfo>BBa_J04450</partinfo>-2 (E-P)
- MyYeast-3 (DNA already available) (E-S)
- <partinfo>pSB4C5</partinfo> (DNA already available) (E-P)
September, 29th
Tecan Test Giacomo
Colony PCR for 6 newly picked colonies.
 I55 colony PCR screening (blank blank 1 ..). No positives nor this time.
Gel run for all digested parts
 Digestions (4C5 myYeast3 I4 I53 J04450-2 J04450-1 I1). and gel run/cut/purification for:
- 4C5 (E-P): 19,3 ng/ul
- My Yeast 3 (E-S): 20,8 ng/ul
- I1: 3,8 ng/ul
- I4 (E-S): 14,2 ng/ul
- I53 (E-X): 21,6 ng/ul
- <partinfo>BBa_J04450</partinfo> (pLac-RBS-mRFP-TT) (E-P): 5,6 ng/ul
- <partinfo>pSB1C3</partinfo>: 10 ng/ul
Ligation of:
| I70=MyYeast 3(E-S)+I4(E-S)
|
| I71=MyYeast 3(E-S)+I1(E-S)
|
| I72=<partinfo>BBa_J04450</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>
|
| I73= I38 (E-S) + I53 (E-X)
|
September, 30th
Transformation of I73 (1ul) into E. coli TOP10. It was plated on LB+Amp 100 ug/ml agar plate.
Since I55 was negative again we decided to ligate it again (probably we had a bad digest for vector - I20 - and a good one for insert - we used I38 in other ligations without problems). We diegested already miniprepped DNA for three hours with 1 ul of enzymes:
Gel run/cut
We repeated I55 ligation using newly digested vector and already digested I38 (E-S)
Inoculum into 5ml LB+Amp of:
- I31
- I35
- I37
- I57
- I60
- INTEIN
- 3x <partinfo>pSB1C3</partinfo>
Cultures were let grow ON at 37°C, 220 rpm.
October, 1st
Transformation of I55 into E. coli DH5-alpha. It was plated on LB+Amp 100 agar plate.
Miniprep and Nanodrop quantification for:
(they were also send sequencing)
- INTEIN
- <partinfo>pSB1C3</partinfo>:
- I37:
- I53:
Digest:
- <partinfo>pSB1C3</partinfo>: E-P
- <partinfo>pSB1C3</partinfo>: E-S
- <partinfo>pSB1C3</partinfo>: X-P
- INTEIN: E-P
- I60: E-S
- I31: S-P
- I35: X-P
- I37: E-X
- I57: E-X
Gel run/cut/extraction/quantification:
(foto)
They were stored at -20°C
ON ligation of:
- IXX: I31 (S-P) + I35 (X-P) (vector <partinfo>pSB1A2</partinfo>)
- INTEIN_C3: INTEIN (E-P) + <partinfo>pSB1C3</partinfo> (E-P)
Pick and inoculum of I73 into 5 ml LB+Amp 100. It was let grow ON at 37°C, 220 rpm.
October, 2nd
|