Team:Calgary/Project/CpxP
From 2010.igem.org

Periplasmic Stress Detectors
What causes periplasmic stress?
Periplasmic stress, also known as envelope stress, is triggered by several factors that influence the ability of the bacteria to communicate with other cells through quorum sensing, pathological pathway, pili formation, outer membrane protein formation which plays a role in adhesion of the cells allowing them to form proper colonies and survive. Many stresses in the periplasmic region are triggered by improper formation of outer protein structures such as pili and lipoprotein disruption that reduces pathogenicity of the bacteria.
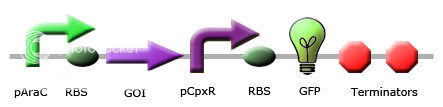
Team Calgary circuits for periplasmic stress detector


|
These images illustrate the promoters of choice by team Calgary for detection of periplasmic stress. The promoters of choice include CpxP, CpxR and DegP promoters. |
How does a native E. coli cell combat periplasmic stress?
E coli have several native sigma factors in the genome that are upregulated during envelope stress. Some of the sigma factors include sigma E and sigma 70. These two factors act upon and induce different proteases and chaperones which act to correct the misfolding agent. There is also a new regulon that is currently studied widely, called the Cpx regulon. The Cpx regulon is known to be activated common agents that increase envelope stress response such as high pH which causes dissolving of membrane and ethanol which is an established protein denaturant. The Cpx activation pathway is
CpxP
CpxP is a member of the Cpx regulon which docks on to CpxA, a transmembrane protein normally. But in the case of envelope stress causing protein misfolding in the periplasmic space, CpxP detaches from CpxA and attaches to the misfolded protein in the periplasmic space.
CpxR
CpxR is a transcription factor that is generally dephoshorylated (inactive form). However, in occasion of misfolded protein in the periplasmic space, CpxR is phosphorylated by CpxA which autophosphorylates itself and also has kinase activity towards CpxR. The phosphorylated CpxR then binds to promoter regions that code for proteases such as DegP, ppiD etc.
DegP
DegP is a protease that is activated by CpxR or sigma70 in the event of envelope stress. DegP is transported into the periplasm where it binds to and degrades the misfolded protein. This allows the CpxP protein to be free resulting in binding to CpxA and shutting the Cpx regulon off. The activation of the Cpx regulon is cyclic in nature and can be reversed when there is a downregulation of activating agents such as misfolded proteins in the cytoplasm.
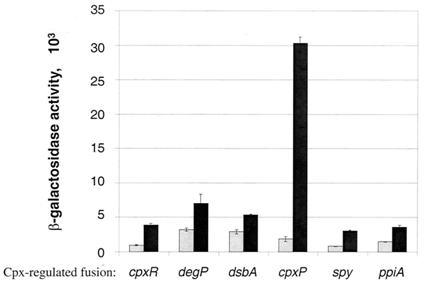
Circuit usage and sensitivity
The circuit that will be constructed to detect protein misfolding will have the cpxP promoter. Two other similar circuits will be constructed to compare the activation of cpxP promoter compared to degP and cpxR promoter. All three promoters have the same function: activation of degP which degenerates misfolded proteins in the cell. As it can be seen in the graph below, the cpxP promoter is the most sensitive to the stress. The purpose of this circuit is to detect protein misfolding. For example, cpxP promoter becomes activated under several specific streses: elevation of pH and overexpression envelope proteins such as NlpE. Hence, if periplasmic misfolding occurs in the cell of an E.coli bacteria, the reporter gene, in this case the Red Fluroscent Protein (RFP), will be activated. Hence, the activation of the RFP will be the indication of a periplasmic protein misfolding.
 "
"