Team:TU Delft/Project/alkane-degradation/results/ADH
From 2010.igem.org
Characterization of the Alcohol DeHydrogenase (ADH) system
Assay based on growth (Alcohols as sole C-source)
- Cultures of our recombinant strain Escherichia coli 018A ([http://partsregistry.org/Part:BBa_K398018 BBa_K398018] on the plasmid pSB1A2) showed no growth, when Octanol-1 and Dodecanol-1 (1% v/v) were used as sole C-source. No further experiments were done using this protocol.
Resting cell assays
- Cells in exponential growth phase were obtained from 50mL cultures. The cells were harevested when the O.D. at 600nm was around 0.3. And the resting cell assays were prepared according to the standard protocol
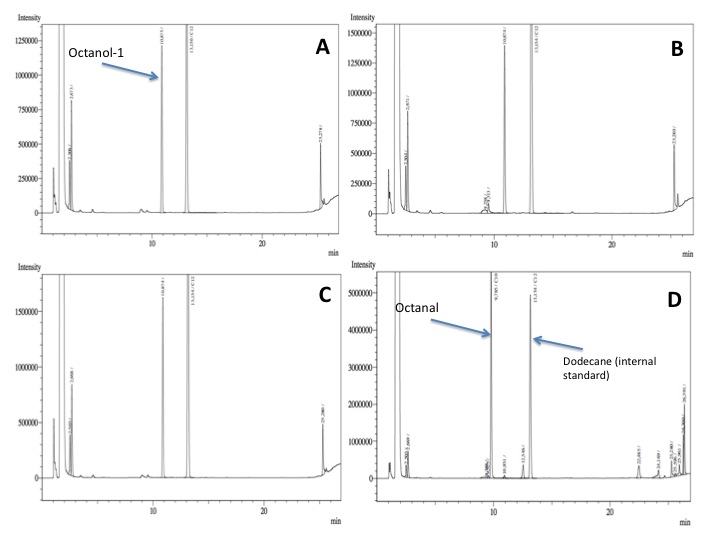
- After an overnight incubation at 37ºC, the organic phase was extracted using 3 mL of ethyl acetate (dodecane was used as internal standard). We performed the GC analysis, the chromatograms are shown below:
- No octanol-1 degradation was found ='( , after this experiment the protocol was abandoned. Probably, the long-chain alcohols cannot pass through the cell membrane and we therefore performed assays with cell extracts in order to know if there was any biological activity in the cytoplasma.
NADH production in cell extracts
- EXPERIMENT 1
Cells were cultured in 50mL of LB medium and harvested when the O.D. 600nm of the culture was around 0.6. Two different cultures of the recominant strain and the negative control were prepared.
Cytoplasmic proteins were extracted using our standard protocol. And a standard curve for protein quantification was prepared.
The total protein of each sample was quantified using 20uL of cell extract.
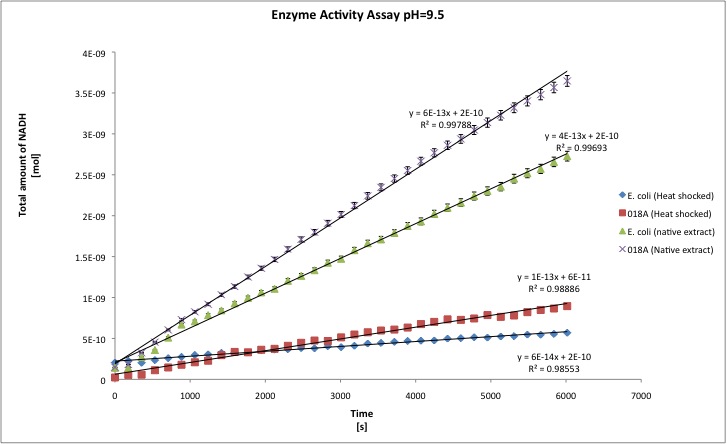
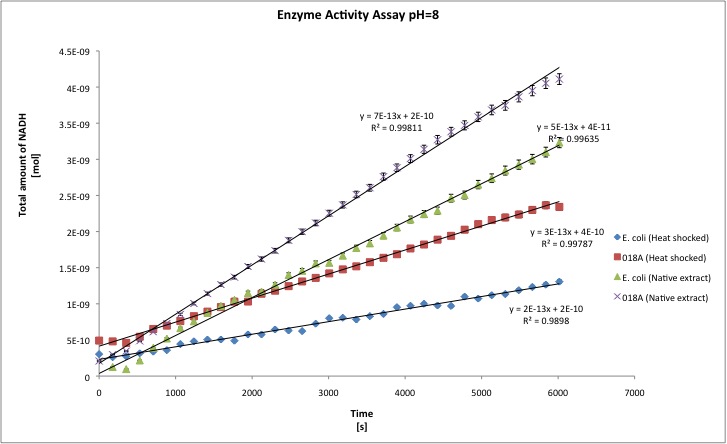
The alcohol dehydrogenase activity was measured using the standard protocol and Dodecanol-1 as substrate. You can download our raw data by clicking on the link: TUDelft_ADH_raw.xls After the data treatment, the results that we obtained are shown in the table below.
- All the enzyme activities were normalized to the TOTAL amount of protein in the cell extract. The results are shown in katal per mg of protein in the cell extract and Enzymatic Units per mg of protein in the cell extract. You can download our results file here: TUDelft_ADH_results.xls
- If you are not familiar with the term katal, [http://www.clinchem.org/cgi/content/full/48/3/586 Click here] for a further explanation.
- T-tests were performed in order to check the statistical significance of the difference between the wild type activity of E. coli as ADH and our recombinant strain. We set our level of significance at 0.025, in two tailed test. That means that if our t-test result is 0.95 we conclude that both samples are statistically different, whereas 0.949 or lower means that both samples are not statistically different.
| pH=9.5 | pH=8 | |||||||
| Heat | Native | Heat | Native | |||||
| J13002 | 018A | J13002 | 018A | J13002 | 018A | J13002 | 018A | |
| kat/mg | 6,156E-12 | 1,941E-11 | 1,053E-11 | 2,806E-11 | 3,211E-12 | 4,496E-11 | 1,302E-11 | 2,638E-11 |
| U/mg | 3,694E-04 | 1,091E-03 | 6,317E-04 | 1,694E-03 | 1,927E-04 | 2,139E-03 | 7,811E-04 | 1,608E-03 |
| stdev | 1,157E-12 | 4,579E-12 | 1,343E-12 | 9,961E-13 | 6,395E-12 | 2,331E-11 | 6,511E-13 | 9,961E-13 |
| Improvement | - | 215,34% | - | 166,51% | - | 1299,87% | - | 166,51% |
| ttest | - | 0,9963 | - | 1,0000 | - | 0,9988 | - | 1,000 |
- EXPERIMENT 2
We ran a second experiment at pH 9.5, with two other strains that seemed to be positive in the colony PCR test. In this test we also added two extra strains of E. coli in order to have more data for the statistical analysis, the strains used were J13002 and 331 ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K398331 BBa_K398331] in pSB1A2). We followed the same procedure as in the experiment 1, for this experiment we also included a positive control which was cell extracts from a culture of Pseudomonas putida growing on octane as sole carbon source, the cells were inoculated the night before and they were harvested when the O.D. at 600 nm was 0.608; the results obtained were the following:
| kat/mg | U/mg | STDEV | Improvement | T-test | Relative to putida | ||
|---|---|---|---|---|---|---|---|
| E. Coli | 8.75E-12 | 5.25E-04 | 8.18987E-12 | - | - | 0.90% | |
| 018A-2 | 9.30E-12 | 5.58E-04 | 1.84877E-12 | 6.26% | 0.4708 | 0.96% | |
| 018A-3 | 3.05E-11 | 1.83E-03 | 6.27802E-12 | 249.14% | 0.9808 | 3.15% | |
| 018A-4 | 8.26E-12 | 4.96E-04 | 1.8256E-12 | -5.59% | 0.4053 | 0.85% | |
| P. Putida | 9.69E-10 | 5.82E-02 | 2.09109E-10 | - | 0.9883 | 100.00% |
According to these results, the other two strains (018A-2 and 018A-4) do not functionally express the part BBa_K398018. However, these results confirm our findings from the first experiment. You can download our result in this link: File:TUDelft ADH results2.xls You can also find a summary in this file:File:TUDelft ADH summary.xls
Conclusions
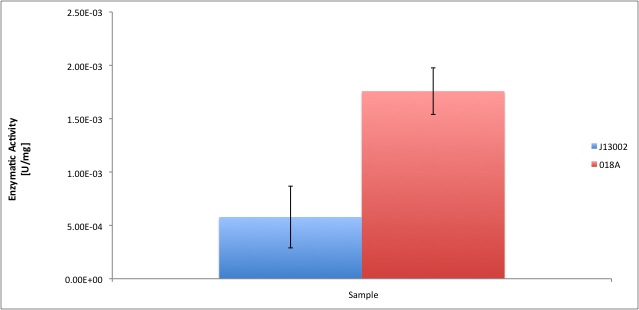
According to our results, the E. coli cell extract has a dodecanol-1 dehydrogenase activity of 9.64e-12 kat/mg (0.58 mU/mg); whereas our recombinant strain 018A has an activity of 2.93e-11 kat/mg (1.76 mU/mg). According to our analysis, the enzymatic activities of both strains are statistically different at confidence level of 0.95, which means that the part [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] increases 2 times the alcohol dehydrogenase activity in the cell extract. We can conclude from our data that the parts [http://partsregistry.org/Part:BBa_K398005 BBa_K398005] and [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] have catalytic activity; particularly when we used [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] the enzyme activity of E. coli cell extracts was equivalent to 3% of the in vitro activity of the positive control (Pseudomonas putida).
However, we think that from this comparison of both in vitro activities we can suggest future teams interested in our part [http://partsregistry.org/Part:BBa_K398005 BBa_K398005] to use a stronger promoter-rbs combination; maybe by increasing the amount of protein produced it is possible to get higher in vitro and in vivo activities. Nevertheless, we have to stress that the in vivo activity of the part [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] is still unknown for us, maybe there is a lack of long-chain alcohol transporter proteins or it is necessary to over-express this part in order to see/measure it.

 "
"