|
SEPTEMBER: WEEK 5
September, 27th
Tecan Test
MiniPrep and E-P digest for I62-a, I62-b, I62-c, I62-d, I62-e, I62-f
DNA PCR for MyYeast 1-2-3
Gel run
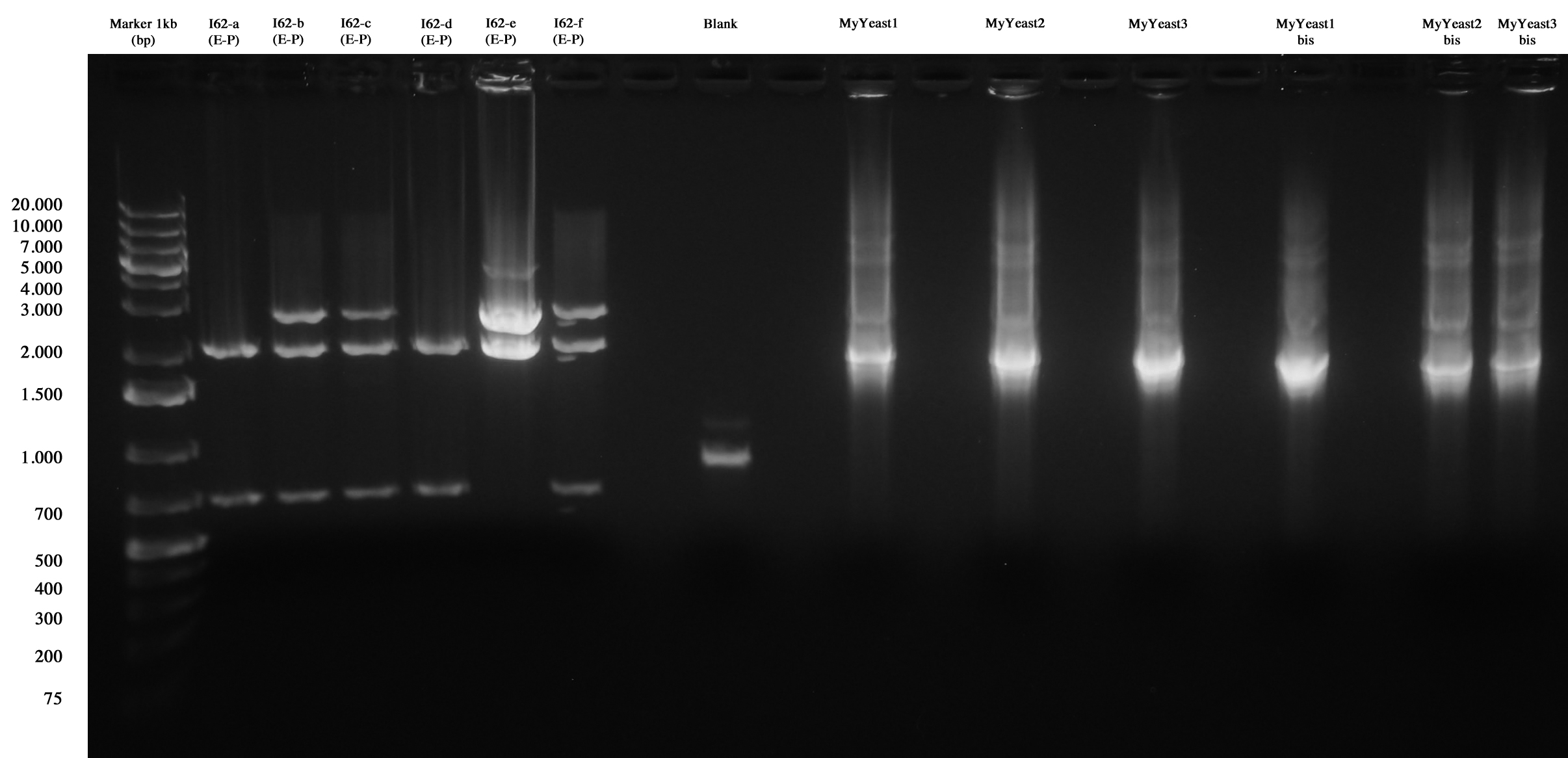 I62 and MyYeast screening.
I62-e was positive, so we made glycerol stock and stored it at -80°C.
Protein electrophoresis for phasins.
September, 28th
Tecan Test
Miniprep and quantification for:
- I1: 59,6 ng/ul
- I4: 109,9 ng/ul
- I53: 160,3 ng/ul
- <partinfo>BBa_J04450</partinfo>-1:55,9 ng/ul
- <partinfo>BBa_J04450</partinfo>-2: 47 ng/ul
ON digestion of:
- I1 (E-S)
- I4 (E-S)
- I53 (E-X)
- <partinfo>BBa_J04450</partinfo>-1 (E-P)
- <partinfo>BBa_J04450</partinfo>-2 (E-P)
- MyYeast-3 (DNA already available) (E-S)
- <partinfo>pSB4C5</partinfo> (DNA already available) (E-P)
September, 29th
Colony PCR for 6 newly picked colonies.
 I55 colony PCR screening. No positives nor this time.
Gel run for all digested parts
and cut/gel purification for:
- 4C5 (E-P): 19,3 ng/ul
- My Yeast 3 (E-S): 20,8 ng/ul
- I1: 3,8 ng/ul
- I4 (E-S): 14,2 ng/ul
- I53 (E-X): 21,6 ng/ul
- <partinfo>BBa_J04450</partinfo> (pLac-RBS-mRFP-TT) (E-P): 5,6 ng/ul
- <partinfo>pSB1C3</partinfo>: 10 ng/ul
Ligation of:
| I70=MyYeast 3(E-S)+I4(E-S)
|
| I71=MyYeast 3(E-S)+I1(E-S)
|
| I72=<partinfo>BBa_J04450</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>
|
| I73= I38 (E-S) + I53 (E-X)
|
Inoculum of MC43, MG43 in 5ml LB with chloramphenicol concentration of 12.5 ug/ml.
September, 30th
Tecan Test
Transformation of I73 (1ul) into E. coli TOP10. It was plated on LB+Amp 100 ug/ml agar plate.
Trasformation of:
- I70 (myYeast with I4, Ptef1-mOrange-tAdh1) in TOP10 and plated on LB+Amp
- I71 (myYeast with I1, mOrange-tAdh1) in TOP10 and plated on LB+Amp
- I72 (pLac-RFP in pSB4C5) in TOP10 and plated on LB+Cm 12,5
All plates were incubated at 37°C ON
Since I55 was negative again we decided to ligate it again (probably we had a bad digest for vector - I20 - and a good one for insert - we used I38 in other ligations without problems). We diegested already miniprepped DNA for three hours with 1 ul of enzymes:
Gel run/cut/extraction/quantification
I20 E-X: 13,4 ng/ul
We repeated I55 ligation using newly digested vector and already digested I38 (E-S)
I55: I38 (E-S) + I20 (E-X)
Inoculum into 5ml LB+Amp of:
- I31
- I35
- I37
- I57
- I60
- INTEIN
- 3x <partinfo>BBa_J04450</partinfo> (to take <partinfo>pSB1C3</partinfo>, so <partinfo>pSB1C3</partinfo> from now on)
Cultures were let grow ON at 37°C, 220 rpm.
Inoculum of E. coli TOP10 in 1,5 ml LB, grown ON at 37°C 220 rpm. Tomorrow we will prepare competent cells!!
Inoculum of:
- S1 in 500ul M9+Cm 12,5
- I47-S1 in 500ul M9+Amp+Cm 12,5
- GFP-S1 in 500ul M9+Amp+Cm 12,5
- RBS32 in 500ul M9+Amp
Cultures were grown ON at 37°C 220 rpm.
Tomorrow we will test the ability of these strains to produce GFP and to lyse when HSL is added.
In order to excise the chloramphenicol resistance cassette from the integrants in E.coli genome we transformed the cells with a plasmid (pCP20 plasmid) carrying the gene encoding for the flippase protein. This protein is able to cut fragment between two FRT flanking sequences. pCP20 was transformed in MC43-abc and MG43-abc and plated on LB+Amp agar plate.
October, 1st
This morning, we checked tranformed plates:
- I72 showed NO colony!! :/ we will repeat this ligation next week
- I70 and I71 showed colonies, 5 for each plate were picked and inoculated in 1ml LB+Amp to prepare glycerol stocks and for screening.
- I73 showed colonies; six of them were picked and inoculated into 5 ml LB+Amp and let grow ON at 37°C, 220rpm in order to do E-P screeening the following day.
Transformation of I55 into E. coli DH5-alpha. It was plated on LB+Amp 100 agar plate and let grow ON at +37°C.
Miniprep and Nanodrop quantification for:
- I31: 76,1 ng/ul
- I35: 66,8 ng/ul
- I60: 279,7 ng/ul
(they were also sent sequencing)
- INTEIN: 123,8 ng/ul
- <partinfo>pSB1C3</partinfo>-1: 104,8 ng/ul
- <partinfo>pSB1C3</partinfo>-2: 141,2 ng/ul
- <partinfo>pSB1C3</partinfo>-3: 113,5 ng/ul
- I37: 174,9 ng/ul
Digest:
- <partinfo>pSB1C3</partinfo>: E-P
- <partinfo>pSB1C3</partinfo>: E-S
- <partinfo>pSB1C3</partinfo>: X-P
- INTEIN: E-P
- I60: E-S
- I31: S-P
- I35: E-P
- I35: E-X
- I37: E-X
- I57: E-X
Gel run/cut/extraction/quantification:
 Intein, I31, I35, I37, I57, I60 digestions |  <partinfo>pSB1C3</partinfo> digested E-P (both vector and insert were kept), E-S, X-P. |
- I37 (E-X): 20,4 ng/ul
- I57 (E-X): 17,6 ng/ul
- I60 (E-S): 9,5 ng/ul
They were stored at -20°C.
- I31 (E-S): 5,4 ng/ul
- I35 (E-P): 1,0 ng/ul ;-(
- I35 (E-X): 14,4 ng/ul
- <partinfo>pSB1C3</partinfo> (E-P): 14,3 ng/ul
- <partinfo>pSB1C3</partinfo> (E-S): 15,2 ng/ul
- <partinfo>pSB1C3</partinfo> (X-P): 13,9 ng/ul
ON ligation of:
- I74: I31 (E-S) + I35 (E-X)
- INTEIN_C3: INTEIN (E-P) + <partinfo>pSB1C3</partinfo> (E-P)
October, 2nd
PCR from colony for I55 (eleven colonies were picked).
Gel run:
 I55 colony PCR screening. We decided to make glycerol stock for I55-1. Inoculum into 5 ml LB+Amp of the positive culture that was let grow ON at 37°C, 220 rpm.
Miniprep and quantification for I73-1/2/3/4/5/6:
- I73-1: 154 ng/ul
- I73-2: 177 ng/ul
- I73-3: 154,7 ng/ul
- I73-4: 145,5ng/ul
- I73-5: 152,6 ng/ul
- I73-6: 154,2 ng/ul
1,5 hours E-P digest (for screening) and gel run
 I70, I71, I73, A9_4C5, A99_4C5 screening. As you can see they are (except for I73-6) all positive. So we stocked I73-1 and stored it at -80°C.
Transformation of I74 and INTEIN_C3 (1ul) in E. coli TOP10. They were plated on LB+Amp100 and LB+Cm34 agar plates respectively. They were let grow ON at +37°C.
Inoculum of I55-1 into 5 ml LB+Amp. ON growth at +37°C, 220 rpm.
October, 3rd
Glycerol stock for:
Pick of a single non-red colony of INTEIN_C3 and inoculum into 5 ml LB+Cm34. Sample was let grow ON at +37°C, 220 rpm.
Miniprep and quantification of:
- I55-1: 57,4 ng/ul
- I55-1bis: 79,6 ng/ul
I55-1bis was digested E-S for about three hours and than gel run/cut.
Colony PCR for I74-1..6.
Gel run for PCR samples and I55 (E-S) and cut for this one.
 I74 screening and I55 E-S cut. Very bad PCR. We performed a massive PCR from colony during the night (I74-7..24), we ran it the following day.
Gel extraction and quantification:
Ligation of I55 (E-S) with already available DNA:
- I75: I55 (E-S) + I37 (E-X)
- I77: I55 (E-S) + I57 (E-X)
Inoculum of I35 (last quantification after gel extraction was too poor; we will digest it again E-P) and I54 into 5 ml LB+Amp: ON growth at +37°C, 220 rpm.
|
|