Team:Washington/Gram Negative/Design
From 2010.igem.org
Type Six Secretion System
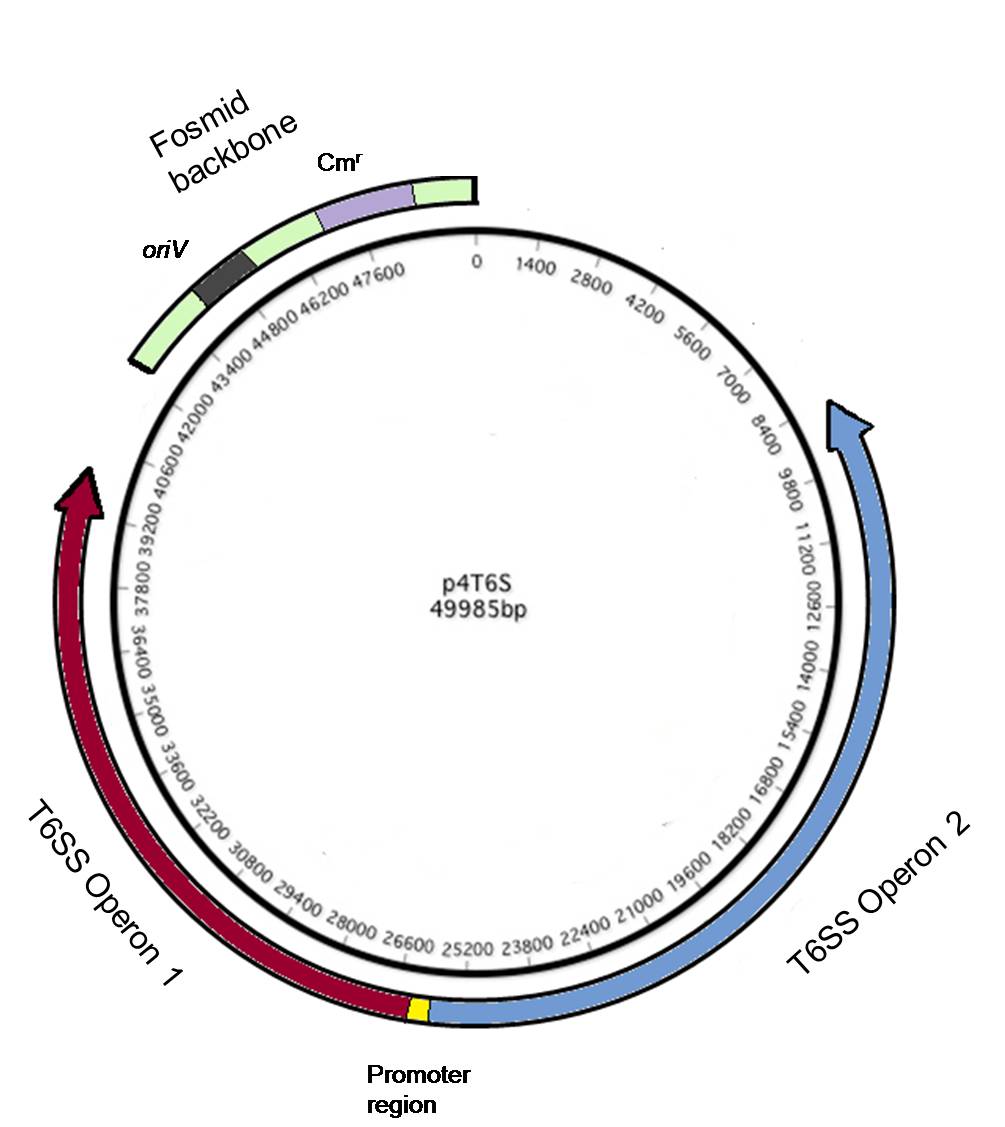
Using a fosmid to transfer the T6SS genes into E. coli
The T6SS is comprised of 23 genes on several operons. Capturing and moving the genes via standard restriction digest cloning would be difficult and impractical. We were made aware that the sequence of P. aeruginosa strain we are using was solved using fosmids (essentially larger plasmids). Luckily, one of the fosmids contained all of the necessary T6SS genes. This allowed for easy transfer of the genes required for a T6SS into E. coli. However, it is not certain that the genes will express in E. coli. In order to verify expression of the T6SS genes in E. coli we performed a western blot using antibodies against Hcp1, a critical protein of the secretion system and a reporter for its activity.
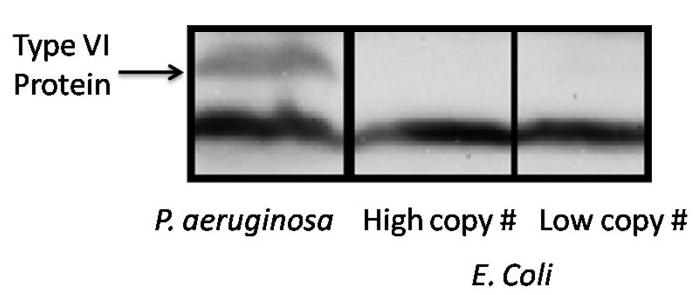
Testing the native Pseudomonas aeriginosa promoter in E. coli
The fosmid was tranfered into E. coli, and the cells were pelleted and western blotted for Hcp, one of the proteins of the "shaft" of the T6SS in order to determine if the genes in the T6SS were being expressed from the native P. aeriginosa promoter. The western blot showed no bands ( figure x), indicating that E. coli was not transcribing from the native P. aeriginosa promoter, and the promoter would need to be changed to a bidirectional promoter compatible with E. coli. It was decided that the native promoter would be switched to a bidirectional T7 promoter, as T7 promoters are well characterized, and known to be highly robust.
Tse2/Tsi2 Toxin/ Antitoxin System
Goal
The purpose of the Tse2/Tsi2 toxin-antitoxin circuit is to regulate the probiotic so that it is usefull in an anti-gram (-) probiotic application. If our probiotic system were constantly producing Tse2 and killing gram (-) cells, there would be an increased risk of the development of tse2 resistance, and the helpful gut flora would be adversly affected. It would be much preferable to activate the T6SS/Tse2/Tsi2 system only when a pathogen is present. While it is possible to regulate the probiotic system by regulating the expression of the T6SS, the response time of such a system would be limited by the complex, 23 protein nature of the T6SS. Therefore, it would be much preferable to activate our probiotic by inducing Tse2 expression only when a pathogen is present.
Design of our Inducable Tse2/Tsi2 system
Activating Tse2 production when a pathogen is present would require a promoter inducable by some molecular stimulis unique to a specific pathogen. In addition, the expression of Tsi2 would need to be constituitve, or induced by the same stimulis that induces Tse2 expression. As a proof-of-concept, this project uses the LuxR-pLux transcription factor- promoter system from Vibrio fischeri to regulate expression of the Tse2-Tsi2 locus. V. fischeri excretes 3OC6HSL, a small membrane permeable molecule( hereafter refered to as HSL). HSL binds to LuxR, changing the conformation of LuxR, which then induces the pLux promoter. Since V. fischeri also produces HSL, expression from the pLux promoter is linked to cell density. This is referred to as quorum sensing. Quorum sensing is found in many pathogenic species, making the use of the pLux-LuxR system a good proof-of concept. When our probiotic detects a gram-negative pathogen-specific molecule ( modeled by HSL), transcription is induced from an inducible promoter( modeled by pLux). This leads to expression of Tse2 ( a toxic protein) and Tsi2 ( its antitoxin). The Type VI Secretion System then attacks the pathogen, puncturing the cell wall. Tse2 is then secreted into the gram negative pathogen, killing the pathogen. This system could easily be changed to target a wide range of gram (-) pathogens by just changing the regulation of the Tse2/Tsi2 locus.
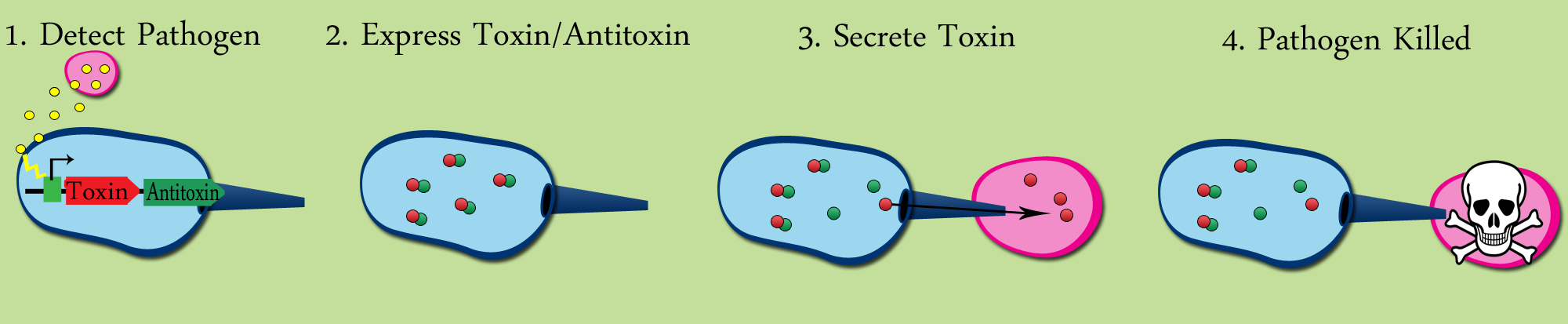
Diagram of the Tse2/Tsi2 HSL inducible circuit
The Tse2/Tsi2 toxin/antitoxin system has a relativly simple circuit design. Tse2 and Tsi2 are present in one operon ( as in Pseudomonas aeruginosa) regulated by the pLux promoter. The LuxR transcriptional factor is constituitively expressed, as no tetR is present to repress the production of LuxR. When HSl is present, it binds to LuxR, resulting the in the induction of Tse2 and Tsi2 production. The pTet, LuxR, and pLux region of the construct is present in part [http://partsregistry.org/Part:BBa_F2620 F2620], easing the contruction of the circuit.

 "
"