August 1-8
In this period(waiting the gene synthesize period), we divided the iGEM works to three parts.
-
- In case of modeling we found hundreds of other antibody sequence for comparing. We found a sequence of antibodies and run a program to find a structure of each antibody. After that we compare the similarity (numbers and figures) with structure alignment program. And we are searching for the mathmetical data that can help us to estimate our system's reaction rate and speed.
- In a meantime, bio brick team finished wiki design. they put contents on bio brick submittion page. We have 6 bio bricks so far and all has uproaded on our wiki page.
- Introduction video of the Presentation
- For presentation team they made a short video. This video will be present before our main presentation for hook. It contains our motivation and our efforts and so on.
August 9
Do list
- structural alignment
- we almost finished structural alignment part. Therefore we need to finish structural alignment part and write it on wiki
- modeling
- We need theoretical data on affinity of our elements through our pathway for modeling. So we need to search on those data.
- main page update
- we decided to put contents that might be confusing when others just look at the menu.
- finishing introduction video
- we are going to take a picture of us for the video
- adding 'tools' menu
- we are making new menu, 'tools'. we will put programs or tools that we used so far.
August 11
do list
- modifying main menu
- taking picture for introduction video
- main page content
- we already finished design we only need contents
- affinity data search
- we only need to find FGFR1-STAT1 Kd data
- putting contents on discoverY wiki page
August 18
We send an e-mail that contains how we want our gene synthesis to be done. And to day we got reply by Bioneer which is our sponsor and helper. There were discussion on how this synthesis can be done and questions are asked on this project. So we gathered our ideas and made a conclusion.
Answers and discussions
- Cloning on S.pombe and cloning in pSB1C3 for submission is necessary things.
- pSB1C3 plasmid information : http://partsregistry.org/Part:pSB1C3:Design
- Part that we will going to put our fusion FGFR in S.pombe http://old.genedb.org/genedb/Search?name=wsc1&organism=pombe&desc=yes&wildcard=yes&searchId=Search
- They offer a method using linker when we are making a Fusion FGFR gene. And we agreed on this idea.
- We decided to use middle expression vector instead of high expression vector for STAT1 cloning. Because high expression of foreign protein can make bad influence to the cell
- There were misunderstanding on GAS promoter. This has to activate only the GFP. (We send our pathway to them for better understanding.)
- Our primer design :
<Back of Fusion FGFR>
- Forward:23bp, Tm 59.
5' - GTTCTGGAAGCCCTGGAAGAGAG - 3'
- Reverse:23bp, Tm 60
3' - TACCGCCTGAGTTTGCGGCGACT - 5'
<Front of Fusion FGFR>
- Forward : 21bp, Tm 58
5' - ATGGTCTTTTTAAATTCCTCT - 3'
- Reverse : 23bp, Tm 59
3' - AGGAGTCGGTTTTGTTGTCGGGG – 5'
<STAT1>
- Forward : 24bp, Tm 59
5' - ATGTCTCAGTGGTACGAACTTCAG - 3'
- Reverse : 24bp, Tm 58
3' - AACTAAAGACACAGACTTCACATT - 5'
PS. we are going to meet tomorrow for gene extracting.
August 19
We went to KIB for gene extraction. Today, as a preparation, we made an LB broth. And we set our experiment table.
- preparing LB broth powder
- adding 500mL of DW on LB broth powder using mass cylinder
- stirring with magnetic bar
- do autoclave
- cooling down LB culture medium
- store it in cylinder (100mL * 2)
- adding ampicillin(1000x) on one of cylinder : 100uL / 100mL
- putting FGFR contain E.coli in ampicillin added cylinder. And adding STAT in other cylinder
- store these two cylinder in 37℃ and 200 rpm condition
Tomorrow we will extract DNA from today's result. And we decided to make a individual experimental note and keep record of our individual works.
August 20
we went to KIB for cell lysis & DNA extraction.
- putting 45mL of STAT1 solution in cornical tube. Also, putting 45mL of FGFR solution in other cornical tube.
- adding 6mL of SolutionI, then vortexing
- adding 6mL of SolutionII, then shaking and rolling
- adding 6mL of SolutionIII, then shaking and rolling
- putting each STAT1, FGFR solution in first column (10 min)
- putting 4mL of equilibrium buffer(QBT) in second buffer
- putting solution that passed by first column in second column
- adding 20mL of washing buffer in second column
- adding 5mL of elution buffer in second column
- adding 5mL of isopropane in second column (5 min)
- pushing the piston for DNA condensation
- adding TE buffer for elution
- checking DNA concentration using machine
File:Result0820 1.jpg
File:Result0820 2.jpg
After DNA extraction, we went to CMS builing for gel electrophoresis.
- preparing 1.2% of agarose gel, 0.5x TAE buffer
- mixing 1.5uL of STAT1, FGFR sample and 1uL of loading buffer
- loading STAT1, FGFR, DNA marker (100V, (-)→(+))
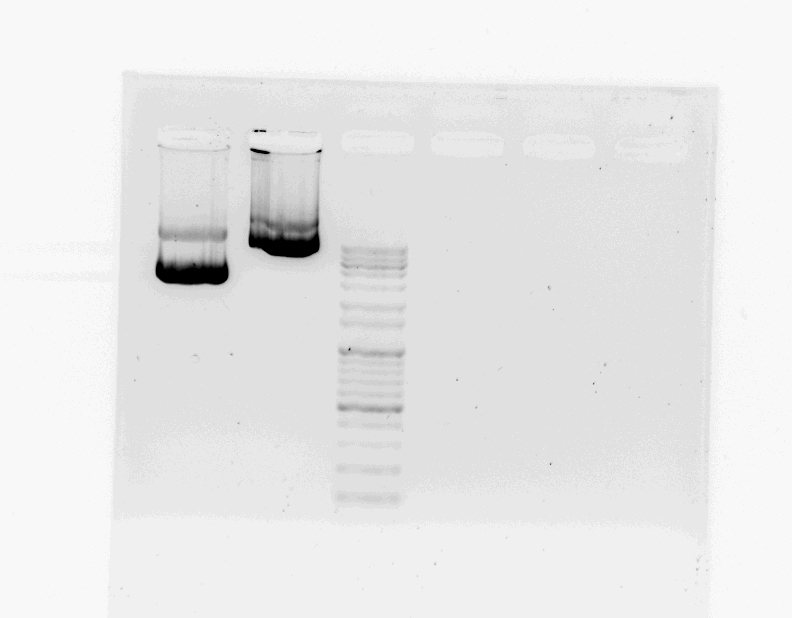
We prefared STAT1, FGFR DNA sample, then we will give these sample to bioneer next week.
|