Team:TU Delft/2 August 2010 content
From 2010.igem.org
Alkane Sensing, Solvent Tolerance and Salt Tolerance
by Pieter
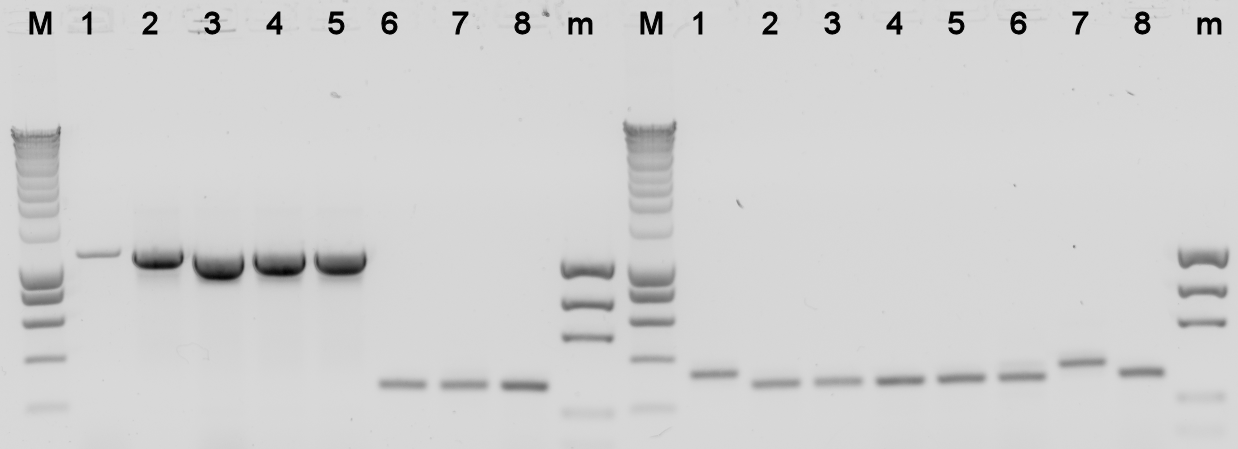
The plates containing yesterday's ligations contained colonies, to check whether they really contain the desired BioBrick a colony PCR was done, and the used colonies were grown in liquid LB medium over night. The results from the PCR were analysed on a 1% agarose gel.
- BBa_K398305 = Alks -> E0240
- BBa_K398101 = bbc1 -> J61101
- BBa_K398402 = PhPFDalpha -> J61101
- BBa_K398403 = PhPFDbeta -> J61101
Lane Description:
| # | Description | Expected Length (bp) | Primers | Status | Remarks |
| M | SmartLadder | n/a | n/a | n/a | |
| 1 | BBa_K398305 | 3772 | G00101 + G00100 | X | |
| 2 | BBa_K398305 | 3772 | G00101 + G00100 | X | |
| 3 | BBa_K398305 | 3772 | G00101 + G00100 | X | |
| 4 | BBa_K398305 | 3772 | G00101 + G00100 | X | |
| 5 | BBa_K398305 | 3772 | G00101 + G00100 | X | |
| 6 | BBa_K398101 | 1007 | G00101 + G00100 | X | |
| 7 | BBa_K398101 | 1007 | G00101 + G00100 | X | |
| 8 | BBa_K398101 | 1007 | G00101 + G00100 | X | |
| m | EZ Ladder | n/a | n/a | n/a | |
| M | SmartLadder | n/a | n/a | n/a | |
| 1 | BBa_K398101 | 1007 | G00101 + G00100 | X | |
| 2 | BBa_K398101 | 1007 | G00101 + G00100 | X | |
| 3 | BBa_K398402 | 836 | G00101 + G00100 | X | |
| 4 | BBa_K398402 | 836 | G00101 + G00100 | X | |
| 5 | BBa_K398402 | 836 | G00101 + G00100 | X | |
| 6 | BBa_K398402 | 836 | G00101 + G00100 | X | |
| 7 | BBa_K398402 | 836 | G00101 + G00100 | X | |
| 8 | BBa_K398403 | 734 | G00101 + G00100 | X | |
| m | EZ Ladder | n/a | n/a | n/a |
Lane Description:
| # | Description | Expected Length (bp) | Primers | Status | Remarks |
| M | SmartLadder | n/a | n/a | n/a | |
| 1 - 7 | Eva's samples | n/a | n/a | n/a | |
| m | EZ tLadder | n/a | n/a | n/a | |
| 1 | BBa_K398403 | 734 | G00101 + G00100 | X | |
| 2 | BBa_K398403 | 734 | G00101 + G00100 | X | |
| 3 | BBa_K398403 | 734 | G00101 + G00100 | X | |
| 4 | BBa_K398403 | 734 | G00101 + G00100 | X | |
| M | SmartLadder | n/a | n/a | n/a |
Emulsifier
When going over the plates in the fridge we noticed a few white colonies on plates from 13-07-'10. Previously we thought there were only red colonies. So we decided to do a colony PCR on all 12 white colonies.
- BBa_K398202 = R0011 + B0032
Lane Description:
| # | Description | Expected Length (bp) | Primers | Status | Remarks |
| M | SmartLadder | n/a | n/a | n/a | |
| 1 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 2 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 3 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 4 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 5 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 6 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 7 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 8 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 9 | BBa_K398202 | 392 | G00101 + G00100 | Isolated plasmid | |
| 10 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 11 | BBa_K398202 | 392 | G00101 + G00100 | ||
| 12 | BBa_K398202 | 392 | G00101 + G00100 | Isolated plasmid |
None of the bands is truly positive. Still number 4,5 and 9 could be positive. Or due to the GC content the bands could be shifted a little bit upwards, so that maybe even sample 12 could be positive.
Just to be sure, we isolated the plasmids and send them to BaseClear for sequencing. The results will be in by Thursday.
Alkane degradation
For the next step in BioBrick formation a digestion was done:
| # | Sample | Enzyme 1 | Enzyme 2 | Enzyme 3 | Buffer | BSA | Needed fragment |
| 1 | 1 μg 007T | EcoRI | SpeI | BamH1 | 2 (Biolabs) | ✓ | ‘E – J61100-AlkB2 – S’ |
| 2 | 1 μg 008A | EcoRI | XbaI | 2 (Biolabs) | ✓ | ‘E – J61100-rubA3 – X’ | |
| 3 | 1 μg 010A | EcoRI | SpeI | AseI | 2 (Biolabs) | ✓ | ‘E – J61100-rubR – S’ |
| 4 | 2 μg B0015 | EcoRI | XbaI | 2 (Biolabs) | ✓ | ‘E – B0015 – pSB1AK3 – X’ | |
| 5 | 1 μg 017A | EcoRI | SpeI | AseI | 2 (Biolabs) | ✓ | ‘E – J61100-ladA – S’ |
| 6 | 1 μg 018A | XbaI | PstI | AseI | 2 (Biolabs) | ✓ | ‘X – J61101-ADH – P’ |
| 7 | 1 μg 019A | EcoRI | SpeI | AseI | 2 (Biolabs) | ✓ | ‘E – J61107-ALDH – S’ |
| 8 | 1 μg pSB1K3 | EcoRI | PstI | 2 (Biolabs) | ✓ | ‘E – pSB1K3 – P’ |
Results of the digestion on 1% agarose gel
The gel was runned at 100V for 1 hour, loaded 5 μL of marker and 5 μL sample + 1 μL loadingbuffer
Lane description:
| # | Description | Expected Length (bp) | Status |
| 1 | Smartladder | ||
| 2 | ‘E – J61100-AlkB2 – S’ (007T) | ✗ | |
| 3 | Undigested 007T | ✗ | |
| 4 | ‘E – J61100-rubA3 – X’ (008A) | ✓ | |
| 5 | Undigested 008A | ✓ | |
| 6 | ‘E – J61100-rubR – S’ (010A) | ✓ | |
| 7 | Undigested 010A | ✓ | |
| 8 | ‘E – B0015 – pSB1AK3 – X’ | ✓ | |
| 9 | pSB1AK3-B0015 | ✓ | |
| 10 | ‘E – J61100-ladA – S’ (017A) | ✓ | |
| 11 | Undigested 017A | ✓ | |
| 12 | ‘X – J61101-ADH – P’ (018A) | ✓ | |
| 13 | Undigested 018A | ✓ | |
| 14 | ‘E – J61107-ALDH – S’ (019A) | ✓ | |
| 15 | Undigested (019A) | ✓ | |
| 16 | ‘E – pSB1K3 – P’ | ✓ | |
| 17 | Smartladder |
 "
"