Team:SDU-Denmark/labnotes4
From 2010.igem.org
Lab notes (8/2 - 8/8)
Contents |
Group: Flagella
Checking if Pst1 site in FlhDC mut is not pressent vol.2
Done by: Sheila & Louise
Date: August 2nd
Protocol: RD1.1
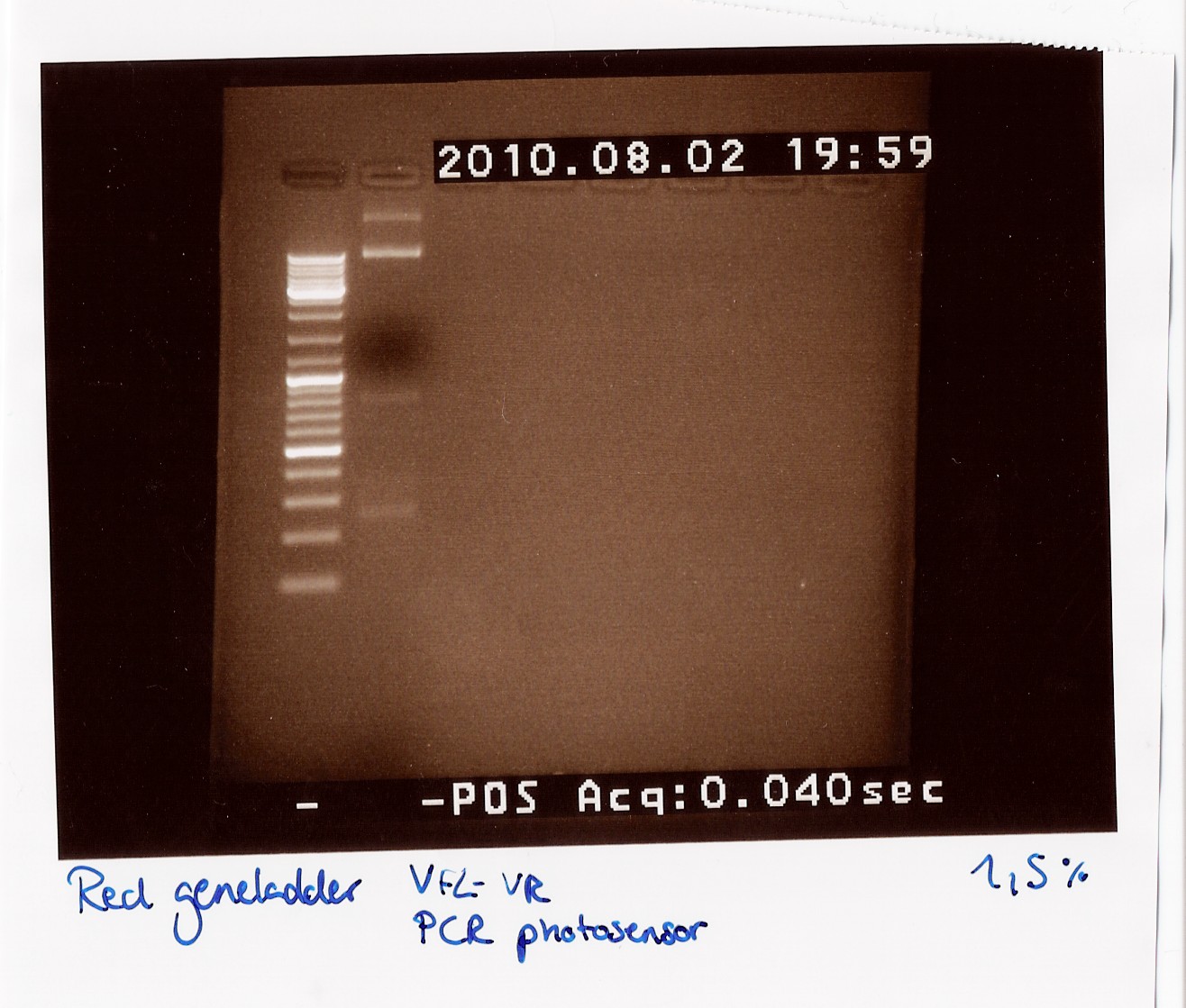
Notes: Freeze tube 9 containing DNA purification from MG1655 cells was used as native FlhDC. Freeze tube 48 containing FlhDC mut purification. These two samples was amplified according to CP1.1 and run with an annealing temperature of 63 celcius and 2 minutes elongation.
The PCR samples were loaded on a1.5% gel with two ladders (100-1000 and 100-10000) FlhDC native was loaded in the first 5 lanes and FlhDC mut in the next 5 lanes.
The gel is expected to show two bands for the native FlhDC gene, one arround 900bp and one arround 100bp, while the FlhDC mut is expected to show only one band, between 900bp and 1000bp.
Results: The gel is pour, shows smear and bands at 200 bp.
300px
We suspect that the primers are to blame for this result. It is possible that when the primer-stock used was made, old primers was used instead of the new longer ones. If this is the case then the annealing temperature of 63 celcius (ajusted to the new primers) is way higher than that of the old ones (45 celcius) which would make it impossible for primers to anneal.
So we made new primer-stock, and removed the old stock and primers.
--Louch07 08:20, 5 August 2010 (UTC)
vol. 3
Done by: Sheila & Louise
Date: August 3nd
Protocol: RD1.1
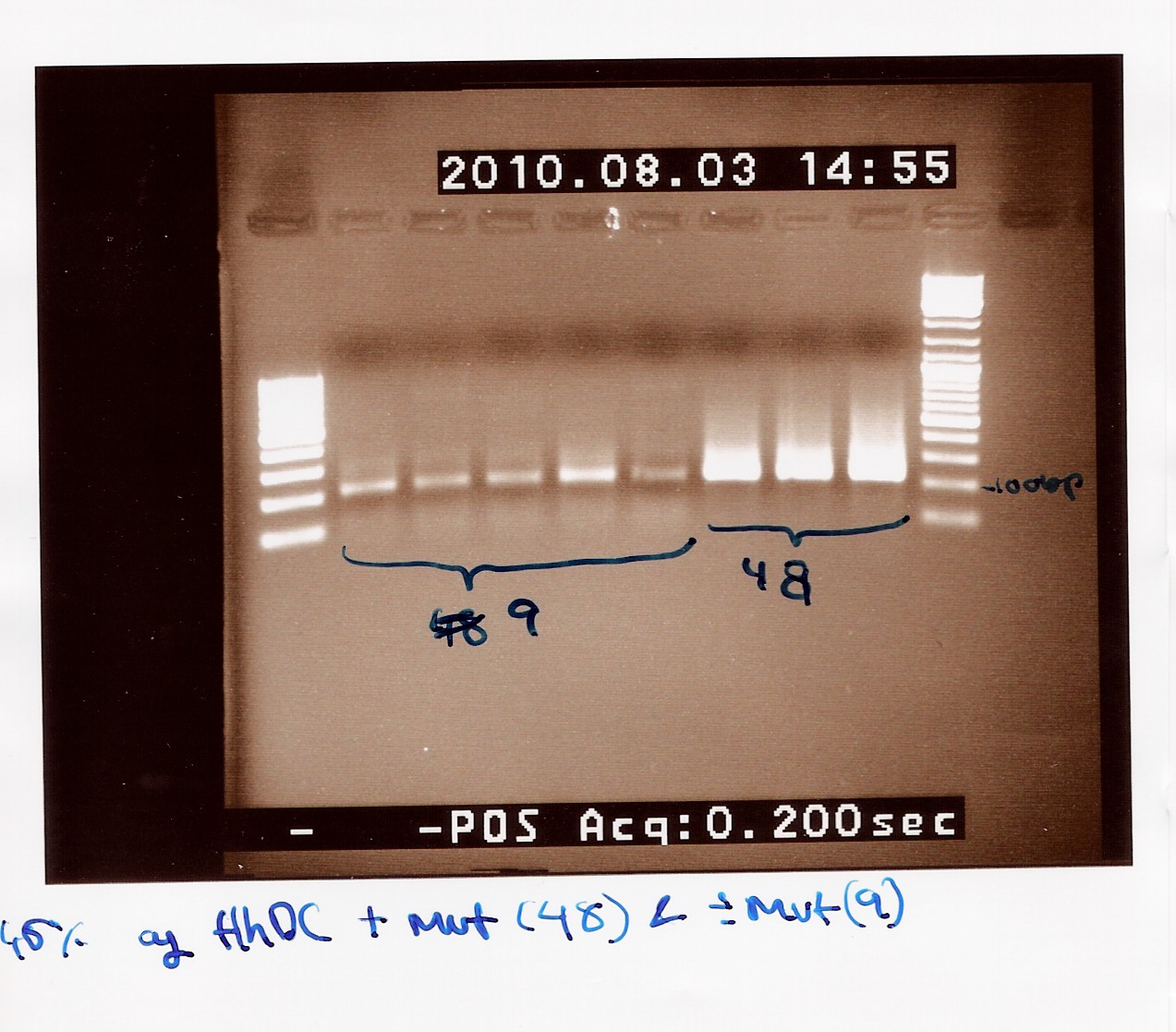
Notes: The previous experiment was redone with the new primers.
Five samples of native FlhDC (FT 9) and 3 samples of FlhDC mut (FT 48) was loaded on a 1.5% gel with two ladders (100-1000 and 100-10000).
Results: The gel still shows smear and bands at 200bp.
We have no explanation this time, and have desided to shelve this experiment a while.
--Louch07 08:20, 5 August 2010 (UTC)
Group: Photosensor
Restriction digest of PUC19 and the photosensor in pKJ606 with Nde1
Start date: 2/08 End date: 2/08
Methods: Restriction digest, gel electrophoresis
Protocol:RD1.1
Experiment done by: LC
Notes: Since all PCR attempts with VF2 and VR had failed until now, we wanted to see if there was an insert at all in the physical DNA we received. Therefore we cut PUC19 and the photosensor to compare their lengths to each other, since the photosensor should be longer than PUC19 uncut and the cut photosensor should consist of two pieces, a 900 - 1000 BP piece and a 3600 BP piece.
Loading order:
1: PUC19 uncut
2: PUC19 cut
3: Photosensor uncut
4: Photosensor cut
Results:
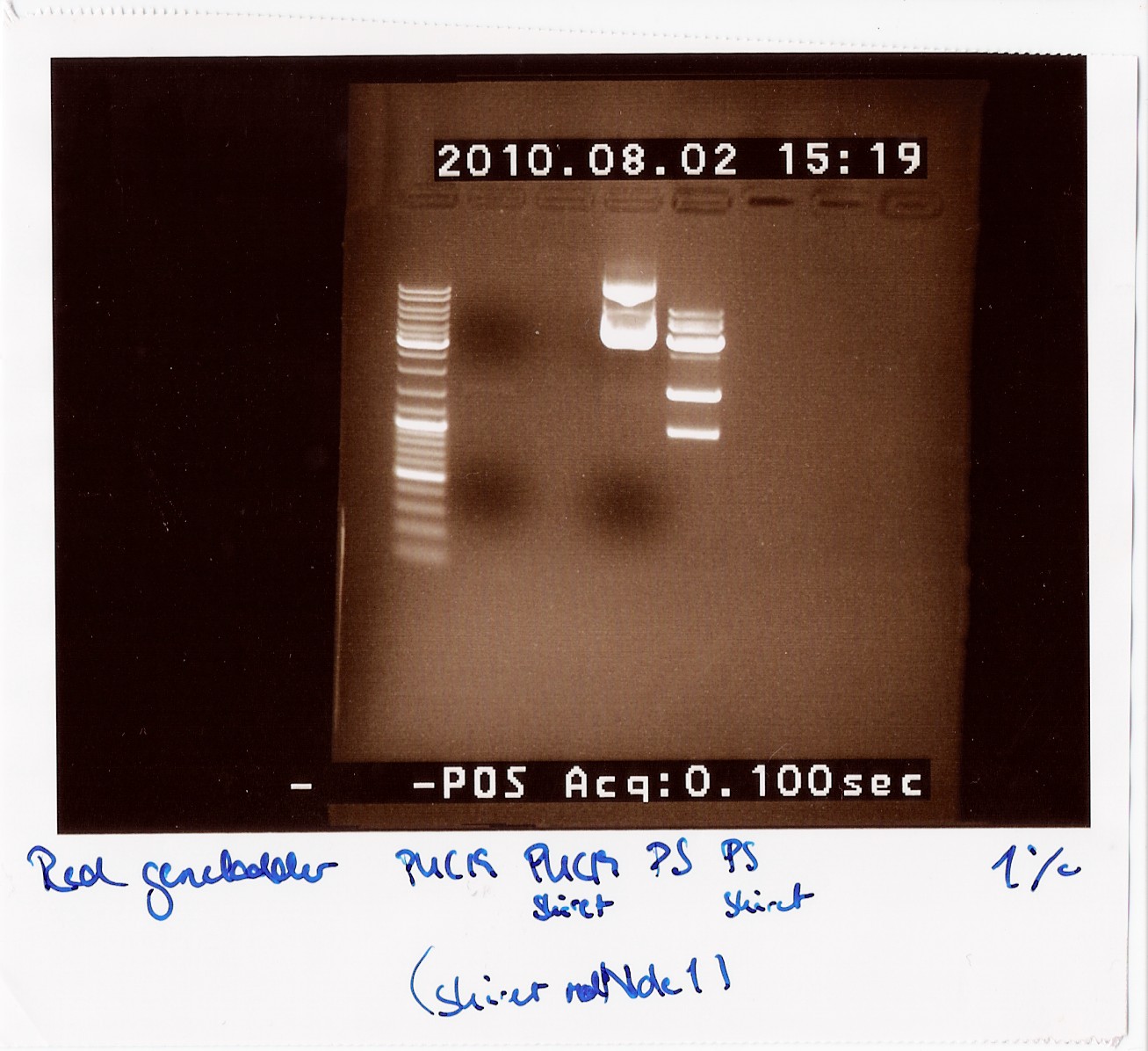
Analysis:
We can clearly see that the photosensor uncut is longer than the uncut PUC19. We can also see multiple bands on the cut photosensor, which are the expected lengths. This indicates that the photosensor gene should be inserted between VF2 and VR, so we are still unsure why the PCR is not working.
Dreamtaq Green PCR Master Mix (2X) PCR on photosensor with VF2 and VR
Start date: 2/08 End date: 2/08
Methods: PCR, gel electrophoresis
Protocol: Dream TAQ Green PCR Master Mix protocol (modified)
Experiment done by: LC
Notes: Instead of a 50 µl reaction we ran a 25 µl reaction. It was mixed like this:
| Dream TAQ Master
Mix |
12,5 µl |
| VF2 |
1 µl |
| VR |
1 µl |
| Template |
0,5 µl |
| Water |
10 µl |
We used a miniprep of the photosensor as template DNA, hence the small amount of template. The PCR program used was as follows:
| Step |
Temperature |
Time |
Number of cycles |
| Initial denaturation |
95° |
1 - 3 min |
1 |
| Denaturation |
95° |
1 min |
30 |
| Annealing |
55° |
1 min |
30 |
| Extension |
72° |
3 min |
30 |
| Final extension |
72° |
3 min |
1 |
Analysis:
We got bands at 270 BP, 850 BP (the distance between VF2 and VR without insert) and two bands very high up in the gel (probably the template which did not get amplified and could not run through the 1,5% gel).
 "
"