Team:Imperial College London/Chassis
From 2010.igem.org
| Chassis |
|
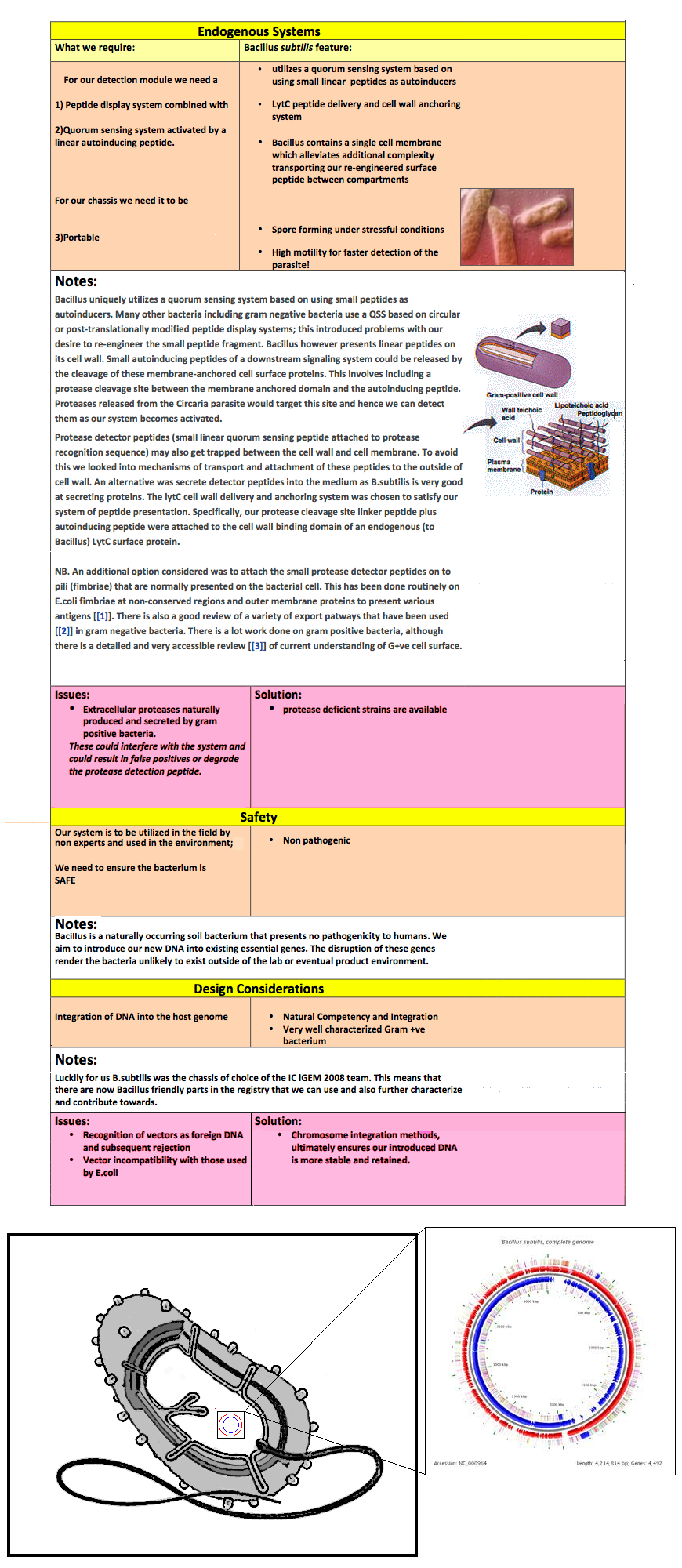
Our choice of chassis was B. subtilis. Here we list the reasons why B. subtilis serves as a more appropriate chassis option with regards to our project design and main project specifications. To re-iterate the pertinent project specifications:
We selected B. subtilis because it is a well characterized gram positive bacterium that is non pathogenic. It was convenient but also a coincidence that within our chosen chassis were features with which we could manipulate to use in our detection module. Below is a brief table summary of how B. subtilis meets our system requirements and also some of the issues we encountered throughout the implementation process and how we overcame them. |
| Bacillus subtilis Breakdown | ||||||||
|
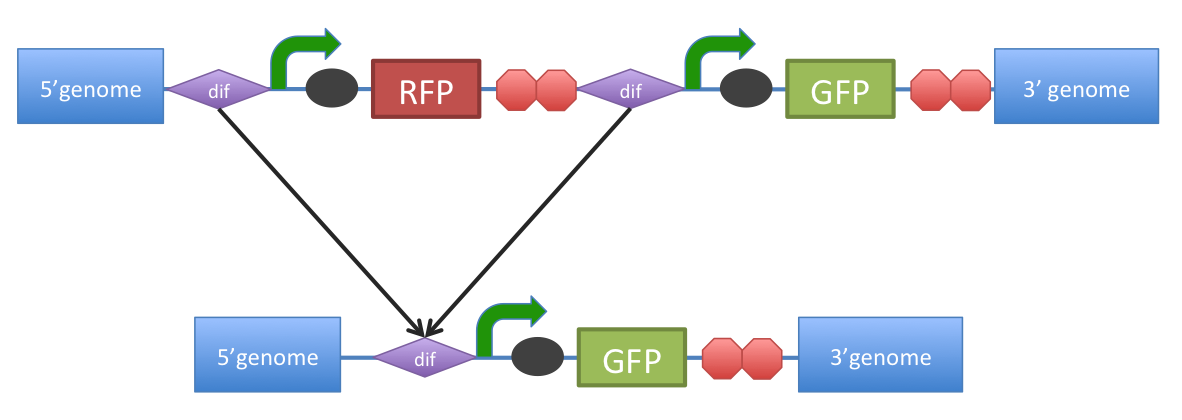
Notes: Bacillus uniquely utilizes a quorum sensing system based on using small peptides as autoinducers. Many other bacteria including gram negative bacteria use a QSS based on circular or post-translationally modified peptide display systems; this introduced problems with our desire to re-engineer the small peptide fragment. Bacillus however presents linear peptides on its cell wall. Small autoinducing peptides of a downstream signaling system could be released by the cleavage of these membrane-anchored cell surface proteins. This involves including a protease cleavage site between the membrane anchored domain and the autoinducing peptide. Proteases released from the Circaria parasite would target this site and hence we can detect them as our system becomes activated. Protease detector peptides (small linear quorum sensing peptide attached to protease recognition sequence) may also get trapped between the cell wall and cell membrane. To avoid this we looked into mechanisms of transport and attachment of these peptides to the outside of cell wall. An alternative was secrete detector peptides into the medium as B. subtilis is very good at secreting proteins. The lytC cell wall delivery and anchoring system was chosen to satisfy our system of peptide presentation. Specifically, our protease cleavage site linker peptide plus autoinducing peptide were attached to the cell wall binding domain of an endogenous (to Bacillus) LytC surface protein. N.B. An additional option considered was to attach the small protease detector peptides on to pili (fimbriae) that are normally presented on the bacterial cell. This has been done routinely on E.coli fimbriae at non-conserved regions and outer membrane proteins to present various antigens 1. There is also a good review of a variety of export patways that have been used 2 in gram negative bacteria. There is a lot work done on gram positive bacteria, although there is a detailed and very accessible review 3 of current understanding of G+ve cell surface.
|
| Safety | ||||||||||||||||||||||
Notes: Bacillus is a naturally occurring soil bacterium that presents no pathogenicity to humans. We aim to introduce our new DNA into existing essential genes. The disruption of these genes render the bacteria unlikely to exist outside of the lab or eventual product environment.
|
 "
"