Team:TU Delft/19 July 2010 content
From 2010.igem.org
Contents |
Forbidden word
Today we chose a word that we were not allowed to say all day. The word was pipet. It turned out to be hard not to use this word. All kind of funny definitions were used to avoid the word pipet. Still some persons accidentally said the word and have to do the dishes tomorrow.
Lab work
Ordered DNA + Solvent Tolerance and Hydrocarbon Sensing
The frozen pellet of last week were isolated using Birnboim protocol.
The following plasmid concentrations were obtained:
| BioBrick | Composed of | Concentration (ng/μL) |
Because not all ligation mixes resulted in transformants were repeated the transformation with the overnight ligated reactions.
Alkane degradation
Biobricks in production: K398007T, K398008T, K398009T, K398010T, K398016T, K398017T & K398018T
Digestion
| # | Sample | Enzyme 1 | Enzyme 2 | Buffer | BSA | Needed fragment |
| 1 | 2 μg J61100 | EcoRI | SpeI | 2 (BioLabs) | ✓ | ‘E–J61100–S’ |
| 2 | 2 μg J61100 | EcoRI | SpeI | 2 (BioLabs) | ✓ | ‘E–J61100–S’ |
| 3 | 1 μg rubR | EcoRI | SpeI | 2 (BioLabs) | ✓ | ‘E–rubR–S’ |
| 4 | 1 μg J61101 | EcoRI | SpeI | 2 (BioLabs) | ✓ | ‘E–J61101–S’ |
| 5 | 1 μg J61107 | EcoRI | SpeI | 2 (BioLabs) | ✓ | ‘E–J61107–S’ |
| 6 | 1 μg alkB2 | Xbal | PstI | 2 (BioLabs) | ✓ | ‘X–alkB2–P’ |
| 7 | 1 μg rubA3 | Xbal | PstI | 2 (BioLabs) | ✓ | ‘X–rubA3–P’ |
| 8 | 1 μg rubA4 | Xbal | PstI | 2 (BioLabs) | ✓ | ‘X–rubA4–P’ |
| 9 | 1 μg B0015 | Xbal | PstI | 2 (BioLabs) | ✓ | ‘X–B0015–P’ |
| 10 | 1 μg ladA | Xbal | PstI | 2 (BioLabs) | ✓ | ‘X–ladA–P’ |
| 11 | 1 μg ADH | Xbal | PstI | 2 (BioLabs) | ✓ | ‘X-ADH–P’ |
| 12 | 1 μg ALDH | Xbal | PstI | 2 (BioLabs) | ✓ | ‘X–ALDH–P’ |
| 13 | 3 μg pSB1T3 | EcoRI | PstI | 2 (BioLabs) | ✓ | ‘E–linear pSB1T3–P’ |
Results of the digestion on 1% agarose gel
Lane description:
| # | Description | Expected Length (bp) | Status | Remarks |
| 1 | Easy load marker | n/a | n/a | |
| 2 | J61100 + EcoRI + SpeI | 78, 2056 | ? | Not visible (too small) |
| 3 | J61100 + EcoRI + SpeI | 78, 2056 | ? | Not visible (too small) |
| 4 | rubR + EcoRI + SpeI | 1227, 2326 | ✓ | |
| 5 | J61101 + EcoRI + SpeI | 78, 2056 | ? | Not visible (too small) |
| 6 | J61107 + EcoRI + SpeI | 78, 2056 | ? | Not visible (too small) |
| 7 | pSB1A2-J23100 + SpeI + PstI | 2110 | ✓ | low concentration |
| 8 | alkB2 + XbaI + PstI | 1255, 2411 | ✓ | |
| 9 | rubA3 + XbaI + PstI | 198, 2409 | ? | Not visible on picture, but was vaguely seen |
| 10 | rubA4 + XbaI + PstI | 207, 2409 | ? | Not visible on picture, but was vaguely seen |
| 11 | B0015 + XbaI + PstI | 155, 3163 | ? | Not visible (too small) |
| 12 | ladA + XbaI + PstI | 1350, 2915 | ✓ | |
| 13 | ADH + XbaI + PstI | 769, 2411 | ✓ | |
| 14 | ALDH + XbaI + PstI | 1521, 1618, 705 | ✓ | Originally in a Kan backbone which gives an extra fragment of 800 bp when cut, rest of the backbone and the fragment both ~ 1500, so overlap |
| 15 | E0240 + XbaI + PstI | 902, 2053 | ✓ | |
| 16 | pSB1T3 + EcoRI + PstI | 2422, 1350 | ✓ | |
| 17 | SmartLadder | n/a | n/a |
Ligation
The digestion products were ligated over night:
| # | BioBrick | Fragment 1 | Fragment 2 | Recipient vector | Final volume |
| 1 | K398007T | 6 μL ‘E-J61100-S’ | 12 μL ‘X-alkB2-P’ | 1.3 μL ‘E-pSB1T3-P’ | 25 μL |
| 2 | K398008T | 6 μL ‘E-J61100-S’ | 11 μL ‘X-rubA3-P’ | 1.3 μL ‘E-pSB1T3-P’ | 25 μL |
| 3 | K398009T | 6 μL ‘E-J61100-S’ | 12.5 μL ‘X-rubA4-P’ | 1.3 μL ‘E-pSB1T3-P’ | 25 μL |
| 4 | K398010T | 10 μL ‘E-rubR-S’ | 11 μL ‘X-B0015-P’ | 1.3 μL ‘E-pSB1T3-P’ | 25.8 μL |
| 5 | K398016T | 6 μL ‘E-J61100-S’ | 12 μL ‘X-ladA-P’ | 1.3 μL ‘E-pSB1T3-P’ | 25 μL |
| 6 | K398017T | 12.5 μL ‘E-J61101-S’ | 11 μL ‘X-ADH-P’ | 1.3 μL ‘E-pSB1T3-P’ | 30 μL* |
| 7 | K398018T | 12.5 μL ‘E-J61107-S’ | 11.5 μL ‘X-ALDH-P’ | 1.3 μL ‘E-pSB1T3-P’ | 30 μL* |
| 8 | n/a | 10.5 μL ‘X-E0240-P’ | n/a | 4 μL ‘S-pSB1A2-J23100-P’ | 25 μL |
To all samples with an end volume of 25μL (and 25.8μL), 2.5 μL Ligase buffer was added. For those with an end volume of 30μL (indicated by an *) 3.0μL Ligase buffer was added.
Emulsifier
Since last weeks attempt to construct the inducible protomer R0011 with RBS B0032 failed, we decided to take a different approach. Instead of doing the standard 2 parts assembly, we amplify the promoter by PCR digest it and ligate in the plasmid that contains the RBS which has only be cut open at the left side. By doing so we do not risk losing the super small DNA fragments like the RBS. The problem is that we cannot use antibiotics selection. So we need to determine that by colony PCR and sequencing.
PCR Amplification
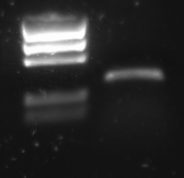
R0011 was amplified with the universal primers G00100 and G00101. The product was put on 1% agarose gel:
Lane description:
| # | Description | Expected length (bp) | Primers | Status | Remarks |
| M1 | BioRad EZ Ladder | n/a | n/a | n/a | |
| 1 | PCR Product of R0011 | 293 | G00100 + G00101 | ✓ | R0011 itself is just 55 bp, but the flanking primer regions are about 100 bp each |
The PCR band is about 300 bps long. R0011 itself is just 55 bp, but the flanking primer regions are about 100 bp each.
Digestion
The PCR product of promotor R0011 was cut with EcoRI and SpeI and the RBS containing plasmid has been cut open with EcoRI and Xbal for 2.5 hours at 37 °C. This deviates from the standard biobrick assembly, thus is not completely as described in our digestion protocol.
| # | Sample | Enzyme 1 | Enzyme 2 | Buffer | BSA | Needed fragment |
| 1 | 1 μg B0032 | EcoRI | Xbal | 2 (BioLabs) | ‘X–B0032 pSB1A2–E’ | |
| 2 | 30 μL PCR product of R0011 | EcoRI | SpeI | 2 (BioLabs) | ‘E-R0011-S’ |
Ligation
The digestion products were ligated over night:
| # | BioBrick | Fragment 1 | Fragment 2 |
| 1 | K398202 | 5 μL ‘X–B0032 pSB1A2–E’ | 10 μL ‘E-R0011-S’ |
Hopefully this will lead to 'E-X-R0011-B0032-S-P' in the B0032 plasmid backbone with Ampicillin resistance marker.
Competent cells
Top10 and DH5α cells were cultivated for making competent cells
Characterization of Anderson RBS sequences
Assembly of reference construct
Friday's ligation products, ligation control and digestion control were transformed into chemically competent Top10 cells and incubated on ampicillin plates.
| # | BioBrick | Plasmid |
| T1 | J23100-B0030-I13401 | pSB1A2 |
| T2 | J23100-B0030-I13401 | pSB1A2 |
| T3 | I13401 (ligation control) | pSB1AK2 |
| T4 | 'E-pSB1A2-I13401-X' (digest control) | pSB1AK2 |
| T5 | MilliQ | - |
Similarly a third attempt was made at transforming K081005, this time into commercial chemically competent cells.
| # | BioBrick | Plasmid |
| T6 | K081005 | pSB1A2 |
In order to continue progress made on the third method, described in the blog of July 14th for the assembly of the reference construct J23100-B0032-E0040-B0015, the following Digestions were performed:
| # | Sample | Enzyme 1 | Enzyme 2 | Buffer | BSA | desired fragment |
| 1 | 1 μg J23100 | SpeI | PstI | 2 (BioLabs) | ‘S–J23100 in J61002–P’ | |
| 2 | 1 μg E0240 + | XbaI | PstI | 2 (BioLabs) | ‘X–E0240–P’ |
After enzyme inactivation, the digestion products were set for ligation overnight.
| # | BioBrick | Fragment 1 | Fragment 2 | Recipient vector | Final volume |
| 1 | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K173000 K173000] | 10.5 μL ‘X-E0240-P’ | - | 4 μL ‘S-pSB1A2-J23100-P’ | 25 μL |
To all samples with an end volume of 25 μL, 2.5 μL ligase buffer was added.
Note: The above method is not exactly the proposed method #3, but a variation without PCR amplification of E0240. This is less elegant; while a re-ligation of the J61002 plasmid will result in colonies with red fluorescence, a re-ligation of the pSB1A2 plasmid containing E0240 cannot easily be screened against (or selected against as both contain ampicillin markers).
In the likelihood that the abovementioned method will not yield desired transformants, a PCR amplification of E0240 was performed in three-fold. The resulting product over 1% agarose gel can be seen below. The PCR amplification product was subsequently digested using XbaI and PstI for later insertion into previously digested (SpeI and PstI) J23100(in J61002).
1% Agarose gel of E0240 PCR product:
Lane descriptions:
| # | Description | Expected Length (bp) | Primers | Status | Remarks |
| 1 | SmartLadder marker | n/a | n/a | n/a | |
| 2 | PCR product of E0240 #1 | 1114 | G00100 + G00101 | ✓ | |
| 3 | PCR product of E0240 #2 | 1114 | G00100 + G00101 | ✓ | |
| 4 | PCR product of E0240 #3 | 1114 | G00100 + G00101 | ✓ |
 "
"