Team:Freiburg Bioware/NoteBook/Labjournal/October2
From 2010.igem.org
(→cloning of lITR_CMV_betaglobin and lITR_phTERT_betaglobin into pSB1C3_CD) |
(→Hannas colony PCR:) |
||
| Line 38: | Line 38: | ||
<b>Investigator: Achim</b><br> | <b>Investigator: Achim</b><br> | ||
| + | |||
| + | |||
| + | |||
| + | ====<p style="font-size:15px; background-color:#66bbff;"><b>Colony PCR of p40_VP123_capins</b></p>==== | ||
| + | <b>Investigator: Bea</b><br> | ||
====<p style="font-size:15px; background-color:#66bbff;"><b>cloning of lITR_CMV_betaglobin and lITR_phTERT_betaglobin into pSB1C3_CD</b></p>==== | ====<p style="font-size:15px; background-color:#66bbff;"><b>cloning of lITR_CMV_betaglobin and lITR_phTERT_betaglobin into pSB1C3_CD</b></p>==== | ||
Revision as of 18:41, 11 October 2010
- March (labday 1)
- April (labday 2 - 5)
- May (labday 6 - 17)
- June (labday 18 - 45)
- July (labday 46 - 75)
- August part 1 (labday 76 - 92)
- August part 2 (labday 93 - 106)
- September part 1 (labday 107 - 123)
- September part 2 (labday 124 - 135)
- October part 1 (labday 136 - 145 )
- October part 2 (labday 146 - 155 )
- October part 3 (labday 156 - 166 )
- November (labday 167 - 170 )
- Cellculture
Contents |
146. labday 11.10.2010
Hannas colony PCR:
Investigator: Achim
Colony PCR of p40_VP123_capins
Investigator: Bea
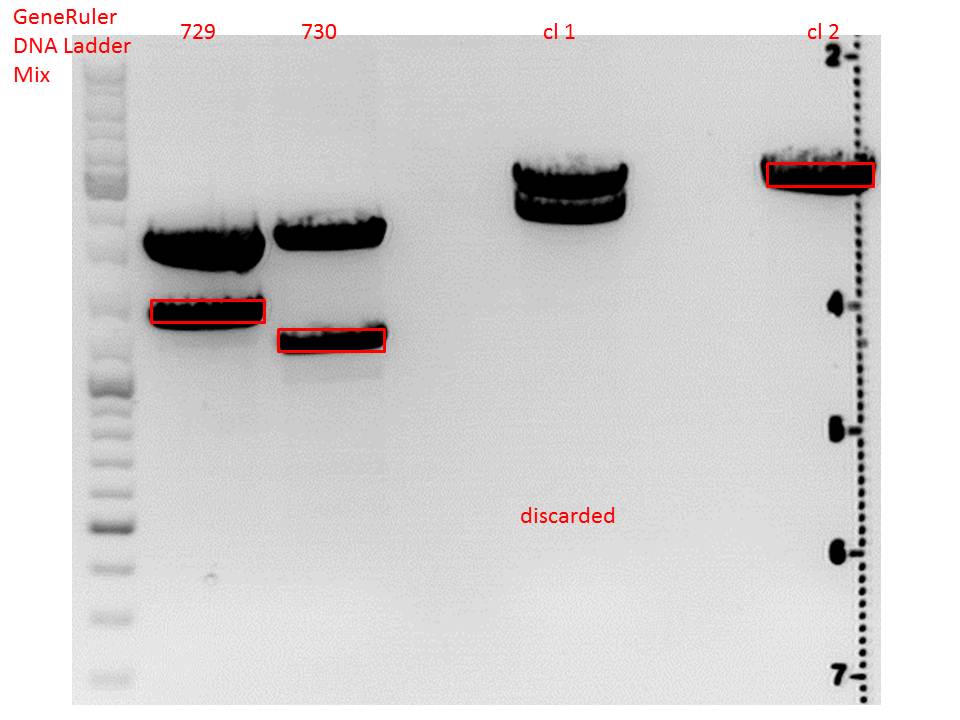
cloning of lITR_CMV_betaglobin and lITR_phTERT_betaglobin into pSB1C3_CD
Investigator: Stefan
Comment: To produce another GOI for testing in cell culture, the cytosine deaminase needs to be assembled with lITR_promotor_betaglobin. In the next step hgH_rITR needs to be added.
Vector name:
pSB1C3_CD clone 1 (P???)
pSB1C3_CD clone 2 (P???)
Insert name:
pSB1C3_lITR_CMV_beta-globin (P729)
pSB1C3_lITR_phTERT_beta-globin (P730)
Digestion:
| components | volume CD clone 1 + 2 /µl | volume P729 /µl | volume P730 /µl |
| DNA | 6 | 14 | 6 |
| BSA (10x) | 2 | 2 | 2 |
| Buffer 4 (10x) | 2 | 2 | 2 |
| Enzyme EcoI | 1 | 1 | 1 |
| Enzyme XbaI | 1 | - | - |
| Enzyme SpeI | - | 1 | 1 |
| H2O | 8 | - | 8 |
| Total volume (e.g. 15,20,25,30 µl) | 20 | 20 | 20 |
Gel:
0,5 g Agarose, 50 ml TAE (1%), 3 µl GELRED , at 115 Volt
CD clone 1 yielded to bands around 2500 bp to 3000 bp. Since the vector was cut only using EcoRI and SpeI, it was expected to be linearized, not to be cut into two fragments this size. Therefore, this sample was discarded and cloning was continued using CD clone 2.
Gel extraction:
Was performed according to protocol.
T4 Ligation:
| ligation name | 729 + CD cl2 | 730 + CD cl2 |
| volume of vector | 3,67 | 2,82 |
| volume of insert | 4,33 | 5,18 |
| T4 ligase buffer (10x) | 1 | 1 |
| T4 ligase | 1 | 1 |
Transformation:
Was performed according to standard protocol using BL21 cells.
147. labday 12.10.2010: Example Example Example Example Example
148. labday 13.10.2010
149. labday 14.10.2010
150. labday 15.10.2010
151. labday 16.10.2010
152. labday 17.10.2010
153. labday 18.10.2010
154. labday 19.10.2010
155. labday 20.10.2010
 "
"