Team:TU Munich/Lab
From 2010.igem.org
(→In vivo Measurements) |
(→In vivo Measurements) |
||
| Line 5: | Line 5: | ||
=Experiment Design= | =Experiment Design= | ||
| - | ==In vivo Measurements== | + | ==''In vivo'' Measurements== |
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
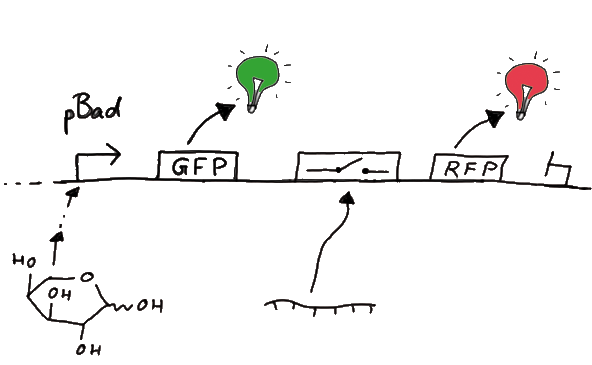
Our initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks ''in vivo'' as well as ''in vitro'', the latter using a translation kit based on <i>E. coli</i> lysate. | Our initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks ''in vivo'' as well as ''in vitro'', the latter using a translation kit based on <i>E. coli</i> lysate. | ||
| Line 12: | Line 12: | ||
In principle we decided to use Green Fluorescent Protein (GFP) as an internal control in front of the switch and another fluorescent protein right after the switch. Since the spectra should not overlap and to avoid FRET as well as an pure overlap of the spectra, we settled on the usage of red fluorescent proteins, namely RFP itself, in the first try. We would use GFP fluorescence as internal control and RFP fluorescence as signal to detect termination/antitermination by our switch we cloned in between the coding sequences of the proteins. Upon binding of the signal the stem loop of the switch would resolve leading to red fluorescent additional to the green one of GFP. The internal control carries the advantage that errors in the measurement set can be detected easily. Lack of arabinose or promotor insensitivity can be recognized as well as problems with the fluorescence measurement itself. Plus, we have a way to normalize our measurements and compare different preparations in relation to each other. | In principle we decided to use Green Fluorescent Protein (GFP) as an internal control in front of the switch and another fluorescent protein right after the switch. Since the spectra should not overlap and to avoid FRET as well as an pure overlap of the spectra, we settled on the usage of red fluorescent proteins, namely RFP itself, in the first try. We would use GFP fluorescence as internal control and RFP fluorescence as signal to detect termination/antitermination by our switch we cloned in between the coding sequences of the proteins. Upon binding of the signal the stem loop of the switch would resolve leading to red fluorescent additional to the green one of GFP. The internal control carries the advantage that errors in the measurement set can be detected easily. Lack of arabinose or promotor insensitivity can be recognized as well as problems with the fluorescence measurement itself. Plus, we have a way to normalize our measurements and compare different preparations in relation to each other. | ||
<br> | <br> | ||
| - | Our measuring plasmid is based on the BioBrick ??, A1, distribution 2010. Unfortunately after two months of cloning we had to recognize that the plasmid in use did not work (see also Biobrick validation--> link). So after the first unsuccessful attempts we had to reclone the system, substituing RFP to mCherry, a dsRED derivative with a spectra in the far red, and adding arabinose inducible promoters in front of both fluorescent proteins. | + | Our measuring plasmid is based on the BioBrick ??, A1, distribution 2010. Unfortunately after two months of cloning we had to recognize that the plasmid in use did not work (see also Biobrick validation--> link). So after the first unsuccessful attempts we had to reclone the system, substituing RFP to mCherry, a dsRED derivative with a spectra in the far red, and adding arabinose inducible promoters in front of both fluorescent proteins. |
| Line 19: | Line 19: | ||
<br> | <br> | ||
| + | To control the expression of the switch (which makes the whole thing switchable), the switch itself is under the control of an IPTG dependent promotor. In the future we want our networks to be able to respond to a variety of external signals like small metabolites, ions or whatever can be found in the parts registry. For a start we went with an established and well-working system like the ''lac''-operon. | ||
| + | <br> | ||
| + | So upon induction with arabinose a rise of GFP expression can be seen. To monitor changes in gene expression we put our E. coli cells in a fluorimeter and measured fluorescent. It worked quite nicely with living cells in a fluorimeter, the only thing to avoid is too much scattering: the cel density should not exceed an OD600 of ???. | ||
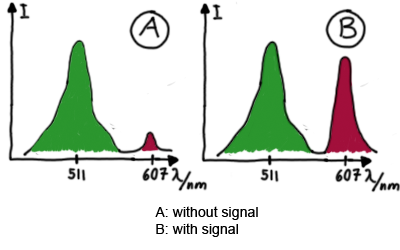
When measuring the termination of our BioBricks and the antitermination by their corresponding signal-RNA, we should be able to observe an increasing RFP emission compared to the GFP emission upon induced signal-RNA production in the cells/in the kit: | When measuring the termination of our BioBricks and the antitermination by their corresponding signal-RNA, we should be able to observe an increasing RFP emission compared to the GFP emission upon induced signal-RNA production in the cells/in the kit: | ||
<br> | <br> | ||
Revision as of 15:58, 11 October 2010
|
||||||||
|
|
Experiment DesignIn vivo Measurements
Our initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks in vivo as well as in vitro, the latter using a translation kit based on E. coli lysate.
To control the expression of the switch (which makes the whole thing switchable), the switch itself is under the control of an IPTG dependent promotor. In the future we want our networks to be able to respond to a variety of external signals like small metabolites, ions or whatever can be found in the parts registry. For a start we went with an established and well-working system like the lac-operon.
Wiith these measurements, it should also be possible to observe differences in efficiency of termination as well as antitermination between our designed switches. In vitro Measurements
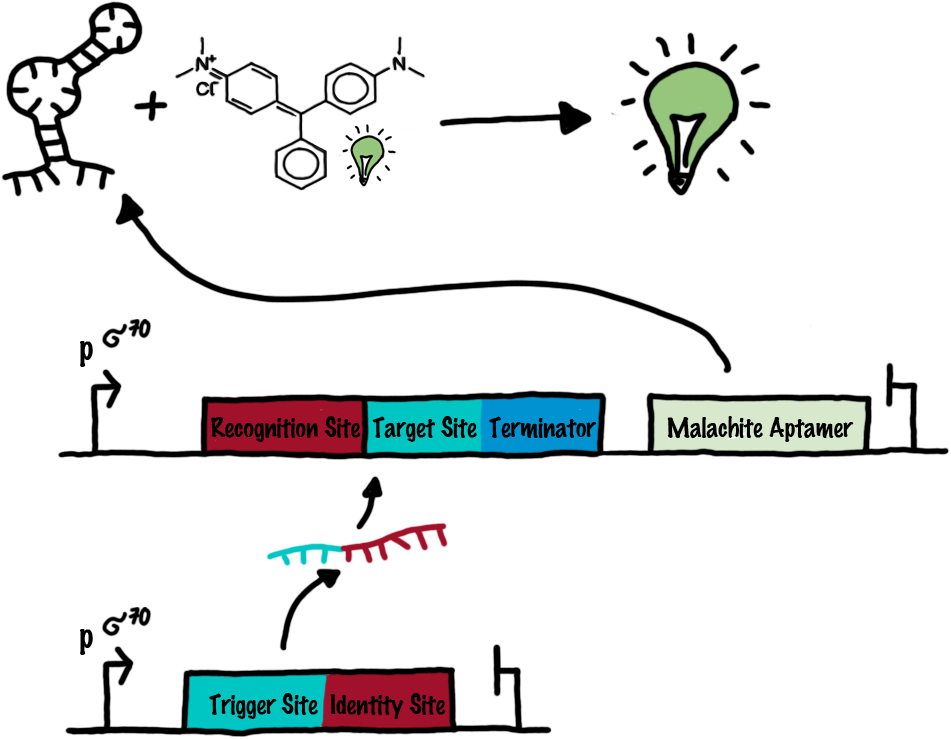
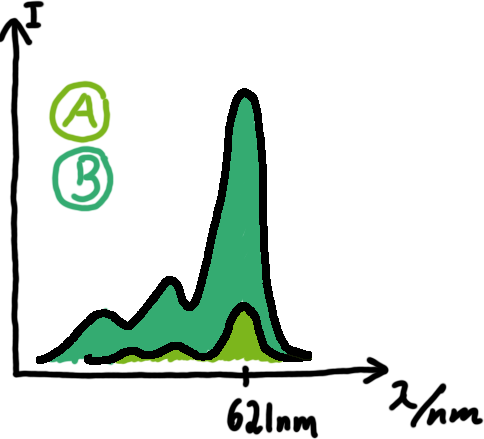
A second possibility to measure parameters of our switches we came up with, was the idea to investigate our system on the transcriptional level only. Therefore, we decided to use malachite green as reporter. Malachite green in a fluorescent dye, whose emission increasing dramaticly (about 3000 times) upon binding of a specific RNA-aptamer.
---concept to be described, as well as literature---
<ref>refs</ref>
To study the switches on the transcriptional level gives the advantage, that we would have less interferences and possible artefacts. Also, we are not sure how cellular mechanisms like degradation of RNases or interacting factors as well as molecular crowding influence our systems. We made constructs comprising of a sigma(70)-binding promoter followed by a short nonsense sequence, the switches and the aptamer sequence. In vitro Translation
So haben wir in vivo translation gemessen Close
ProtocolsMolecular BiologyPCR
So geht ne PCR CloseDigestion
So geht ne PCR CloseIn vivo measurement
So haben wir in vivo gemessen CloseIn vitro measurement
So haben wir in vitro gemessen Close
Lab BookExplanation:
|
|||||||
 "
"