Team:KAIST-Korea/Notebook/Diary/September
From 2010.igem.org
(Difference between revisions)
(→9/10 ~ 9/12 PCR) |
(→September 10 ~ September 12) |
||
| Line 2,007: | Line 2,007: | ||
[[Image:KAIST-exp14.png|320px]] | [[Image:KAIST-exp14.png|320px]] | ||
<br> | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | ==September 13 ~ September 16== | ||
| + | |||
| + | <span style=font-size:12px><b>PCR composition </b></span> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td><b>Materials</b></td><td><b>Amount</b></td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td>Template</td><td>100pmole</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td>Primer A</td><td>50pmole</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td>Primer B</td><td>50pmole</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td>Bioneer Accupower HF PCR premix </td><td> < 1ul</td> | ||
| + | </tr> | ||
| + | <tr align=center><td>DW (3rd sterile water)</td><td>rest</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td>Total</td><td>50ul</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | <br> | ||
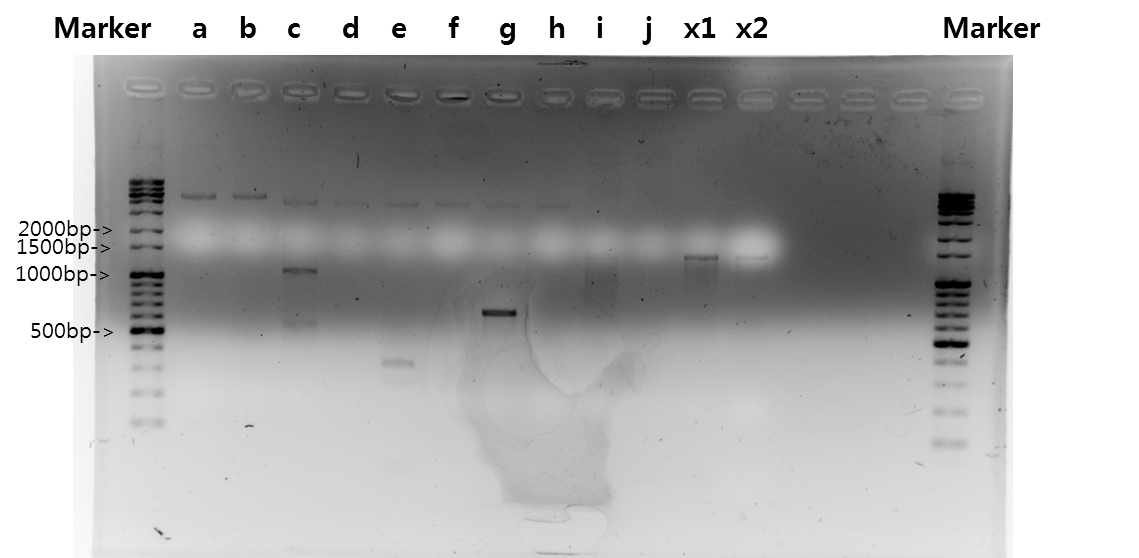
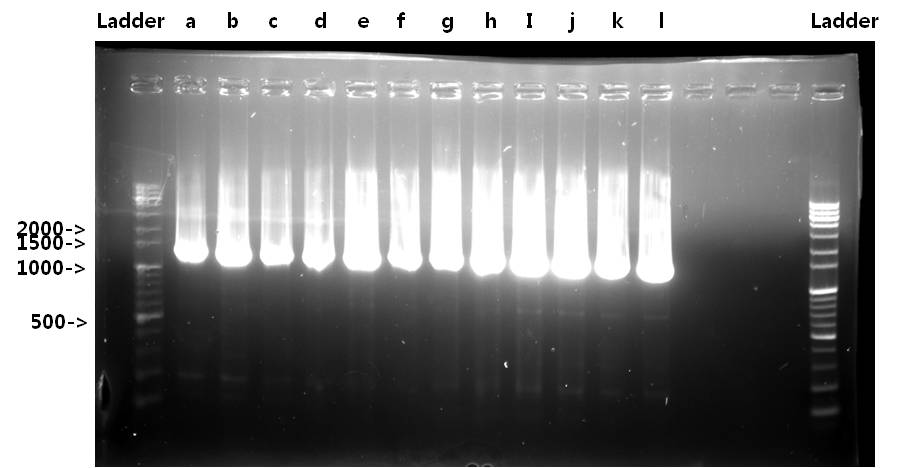
| + | <b>Exp 1</b> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "300px"><b>Segment</b></td><td width = "200px"><b>Annealing Temperature</b></td><td width = "300px"><b>Result</b></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">a</td><td width = "255px">STAT1</td><td width = "200px">50*C</td><td width = "223px">100%</td><td width = "70px">2139bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">b</td><td width = "255px">STAT1</td><td width = "200px">52*C</td><td width = "223px">100%</td><td width = "70px">2139bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">c</td><td width = "255px">STAT1</td><td width = "200px">54*C</td><td width = "223px">100%</td><td width = "70px">2139bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">d</td><td width = "255px">STAT1</td><td width = "200px">56*C</td><td width = "223px">100%</td><td width = "70px">2139bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">e</td><td width = "255px">STAT1</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">2139bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">f</td><td width = "255px">STAT1</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">2139bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">g</td><td width = "255px">V_STAT1</td><td width = "200px">50*C</td><td width = "223px">100%</td><td width = "70px">2151bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">h</td><td width = "255px">V_STAT1</td><td width = "200px">52*C</td><td width = "223px">100%</td><td width = "70px">2151bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">i</td><td width = "255px">V_STAT1</td><td width = "200px">54*C</td><td width = "223px">100%</td><td width = "70px">2151bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">j</td><td width = "255px">V_STAT1</td><td width = "200px">56*C</td><td width = "223px">100%</td><td width = "70px">2151bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">k</td><td width = "255px">V_STAT1</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">2151bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">l</td><td width = "255px">V_STAT1</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">2151bp</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | <br> | ||
| + | <br> | ||
| + | [[Image:KAIST-exp21.png|700px]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
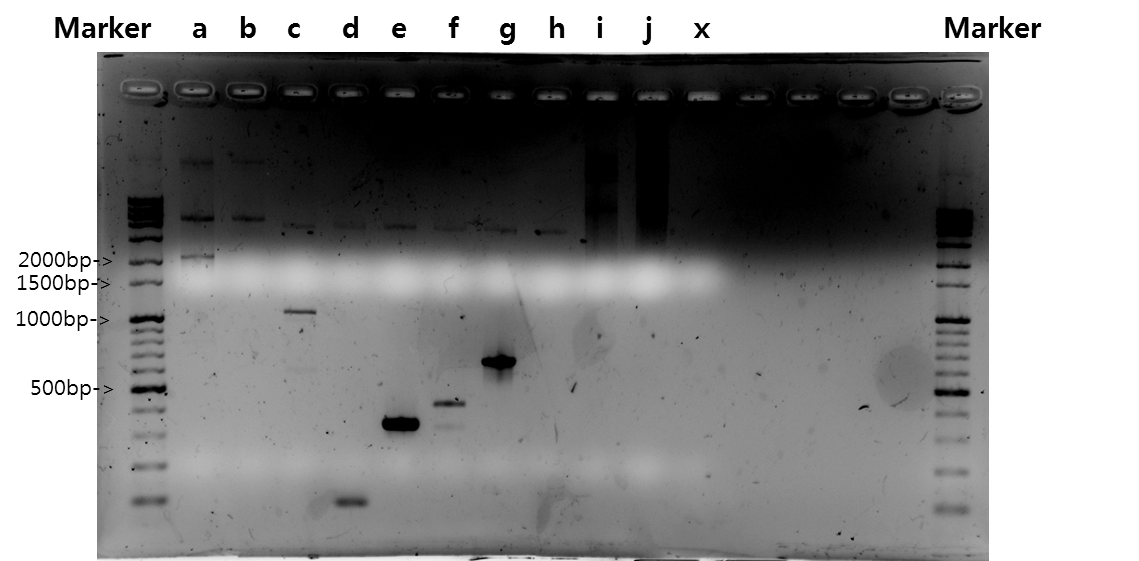
| + | <b>Exp 2</b> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "300px"><b>Segment</b></td><td width = "200px"><b>Annealing Temperature</b></td><td width = "300px"><b>Result</b></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">a</td><td width = "255px">B_FGFR</td><td width = "200px">50*C</td><td width = "223px">100%</td><td width = "70px">1398bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">b</td><td width = "255px">B_FGFR</td><td width = "200px">52*C</td><td width = "223px">100%</td><td width = "70px">1398bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">c</td><td width = "255px">B_FGFR</td><td width = "200px">54*C</td><td width = "223px">100%</td><td width = "70px">1398bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">d</td><td width = "255px">B_FGFR</td><td width = "200px">56*C</td><td width = "223px">100%</td><td width = "70px">1398bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">e</td><td width = "255px">B_FGFR</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">1398bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">f</td><td width = "255px">B_FGFR</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">1398bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">g</td><td width = "255px">VB_FGFR</td><td width = "200px">50*C</td><td width = "223px">100%</td><td width = "70px">1420bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">h</td><td width = "255px">VB_FGFR</td><td width = "200px">52*C</td><td width = "223px">100%</td><td width = "70px">1420bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">i</td><td width = "255px">VB_FGFR</td><td width = "200px">54*C</td><td width = "223px">100%</td><td width = "70px">1420bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">j</td><td width = "255px">VB_FGFR</td><td width = "200px">56*C</td><td width = "223px">100%</td><td width = "70px">1420bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">k</td><td width = "255px">VB_FGFR</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">1420bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">l</td><td width = "255px">VB_FGFR</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">1420bp</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | <br> | ||
| + | <br> | ||
| + | [[Image:KAIST-exp22.png|700px]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
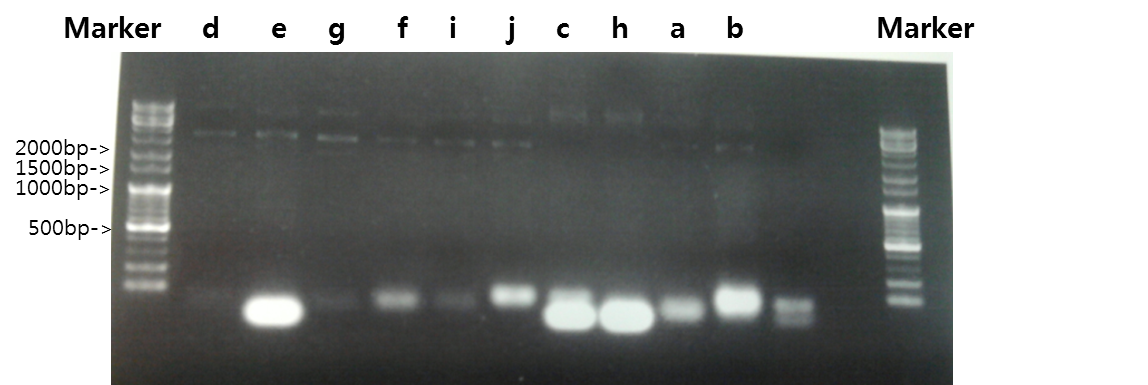
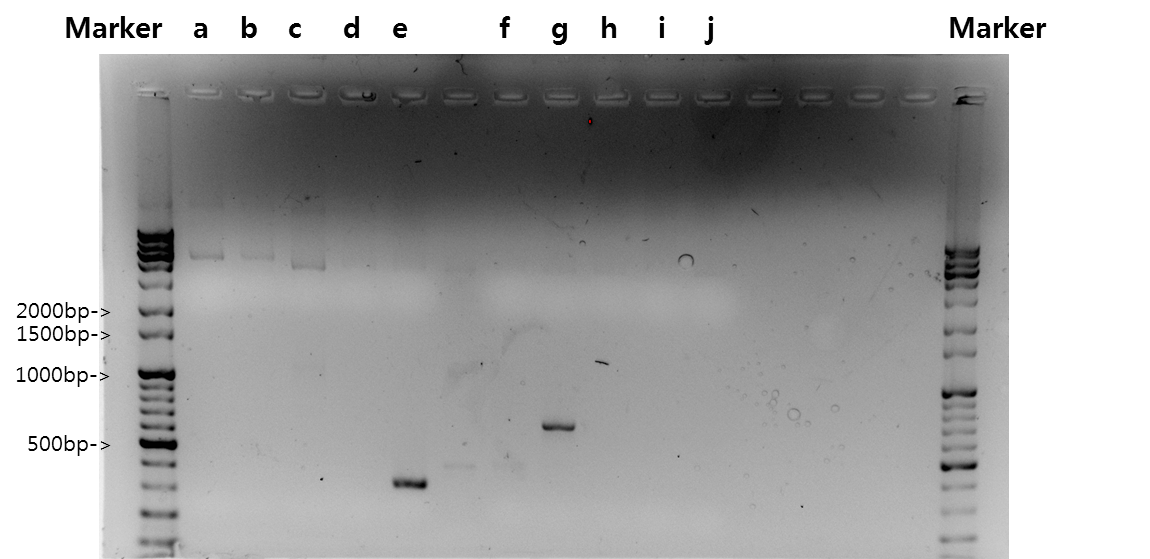
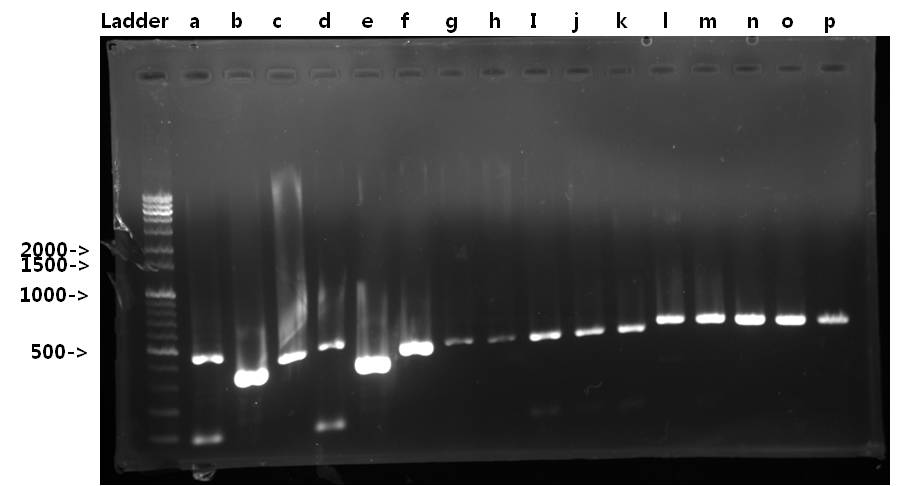
| + | <b>Exp 3</b> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "300px"><b>Segment</b></td><td width = "200px"><b>Annealing Temperature</b></td><td width = "300px"><b>Result</b></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">a</td><td width = "255px">Signal peptide</td><td width = "200px">61*C</td><td width = "223px">30%</td><td width = "70px">87bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">b</td><td width = "255px">Ig-like</td><td width = "200px">61*C</td><td width = "223px">100%</td><td width = "70px">339bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">c</td><td width = "255px">Signal peptide + Ig-like</td><td width = "200px">61*C</td><td width = "223px">100%</td><td width = "70px">426bp</td> | ||
| + | </tr> | ||
| + | <td width = "40px">d</td><td width = "255px">Signal peptide</td><td width = "200px">59*C</td><td width = "223px">30%</td><td width = "70px">87bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">e</td><td width = "255px">Ig-like</td><td width = "200px">59*C</td><td width = "223px">100%</td><td width = "70px">339bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">f</td><td width = "255px">Signal peptide + Ig-like</td><td width = "200px">59*C</td><td width = "223px">100%</td><td width = "70px">426bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">g</td><td width = "255px">3'UTR</td><td width = "200px">52*C</td><td width = "223px">50%</td><td width = "70px">444bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">h</td><td width = "255px">3'UTR</td><td width = "200px">54*C</td><td width = "223px">50%</td><td width = "70px">444bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">i</td><td width = "255px">3'UTR</td><td width = "200px">56*C</td><td width = "223px">100%</td><td width = "70px">444bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">j</td><td width = "255px">3'UTR</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">444bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">k</td><td width = "255px">3'UTR</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">444bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">l</td><td width = "255px">5'UTR</td><td width = "200px">52*C</td><td width = "223px">100%</td><td width = "70px">500bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">m</td><td width = "255px">5'UTR</td><td width = "200px">54*C</td><td width = "223px">100%</td><td width = "70px">500bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">n</td><td width = "255px">5'UTR</td><td width = "200px">56*C</td><td width = "223px">100%</td><td width = "70px">500bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">o</td><td width = "255px">5'UTR</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">500bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">p</td><td width = "255px">5'UTR</td><td width = "200px">69*C</td><td width = "223px">70%</td><td width = "70px">500bp</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | <br> | ||
| + | [[Image:KAIST-exp23.png|700px]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
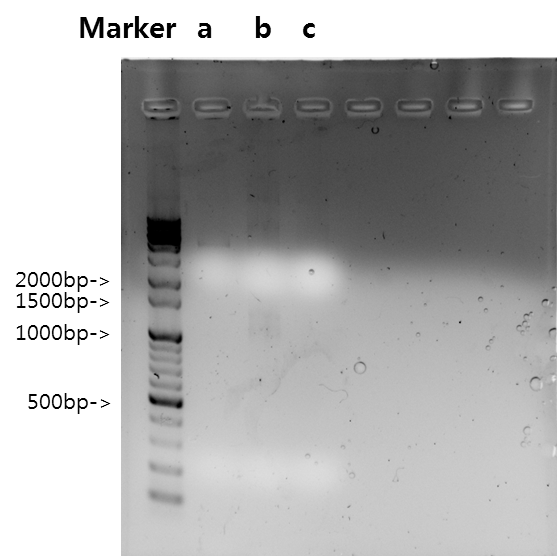
| + | <b>Exp 4</b> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "300px"><b>Segment</b></td><td width = "200px"><b>Annealing Temperature</b></td><td width = "300px"><b>Result</b></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <table border=1> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">a</td><td width = "255px">F_Fusion antibody receptor</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">1095bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">b</td><td width = "255px">F_Fusion antibody receptor</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">1095bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">c</td><td width = "255px">Antibody</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">669bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">d</td><td width = "255px">Antibody</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">669bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">e</td><td width = "255px">VF_Fusion antibody receptor</td><td width = "200px">60*C</td><td width = "223px">100%</td><td width = "70px">1107bp</td> | ||
| + | </tr> | ||
| + | <tr align=center> | ||
| + | <td width = "40px">f</td><td width = "255px">VF_Fusion antibody receptor</td><td width = "200px">58*C</td><td width = "223px">100%</td><td width = "70px">1107bp</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | <br> | ||
| + | <br> | ||
| + | [[Image:KAIST-exp24.png|360px]] | ||
<br> | <br> | ||
<br> | <br> | ||
Revision as of 19:40, 16 September 2010
September 1pREP41 vector has arrived. -> we will do E.coli transformation and increase its number. pREP42-GFP vector has arrived (in E.coli) -> will incubate a day and do spreading for further experiment. Making culture media
Culture
September 2 ~ September 5PCR Setting V_STAT1 (expression)
STAT1 (submission)
Fusion Antibody Receptor (Submission)
Signal Peptide (Submission)
Ig-like (Submission)
Signal Peptide + Ig-like (Submission)
Antibody (Submission)
V_Fusion Antibody Receptor (Expression)
B_FGFR1 (Submission)
VB_FGFR1 (Expression)
Fusion Antibody Receptor (Expression)
5’UTR (expression)
3’UTR (expression)
Selection Marker (expression)
Core promoter + GFP (expression)
Proximal promoter (expression)
HA1 (expression)
HA2 (expression)
Fusion antibody receptor (expression)
5’UTR + Fusion Antibody receptor (expression)
HA1 + Selection Marker (expression)
HA2 + 3'UTR (expression)
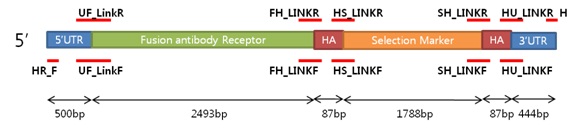
5’UTR - Fusion Antibody receptor + HA1 - Selection Marker (expression)
Homologous Recombinant (expression)
September 6 ~ Septembr 9PCR composition
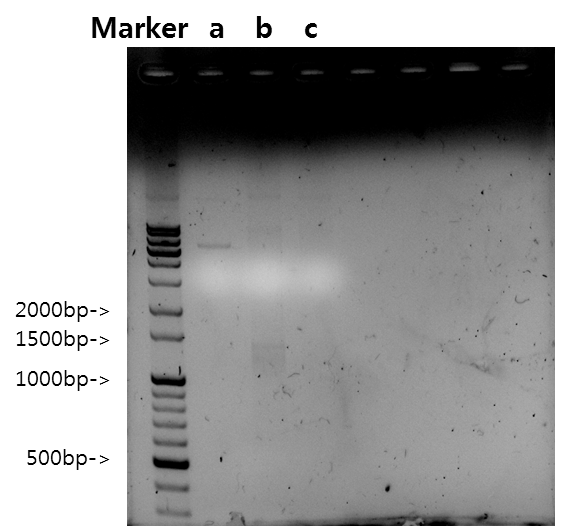
September 10 ~ September 12PCR composition
September 13 ~ September 16PCR composition
|
 "
"