Team:Washington/Gram Positive/Design
From 2010.igem.org
| Line 29: | Line 29: | ||
Naturally produced CapD auto-cleaves itself to create a dimer. The new N terminus contains a critical catalytic threonine residue which allows the enzyme to cut poly-γ-D-glutamate. | Naturally produced CapD auto-cleaves itself to create a dimer. The new N terminus contains a critical catalytic threonine residue which allows the enzyme to cut poly-γ-D-glutamate. | ||
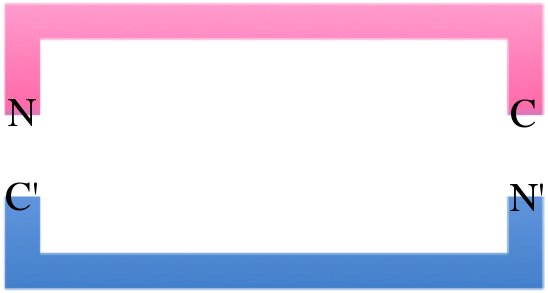
| - | To increase expression and stability, we created a circular permutation of CapD. Typically we view the primary structure of proteins as linear, but when the N and C termini are in close proximity after folding, the protein can be thought of as a circle with a small gap. Frequently, the gap is moved by creating a linker between the naturally occurring N and C termini, then changing the starting and ending residues. This works so long as the amino acid sequence remains the same relative to itself (neighboring amino acids still have the same neighbors). For CapD, we took the site that normally auto-cleaves the N terminus and added a FoldIt designed linker between the natural CapD N and C terminus. This circular | + | To increase expression and stability, we created a circular permutation of CapD. Typically we view the primary structure of proteins as linear, but when the N and C termini are in close proximity after folding, the protein can be thought of as a circle with a small gap. Frequently, the gap is moved by creating a linker between the naturally occurring N and C termini, then changing the starting and ending residues. This works so long as the amino acid sequence remains the same relative to itself (neighboring amino acids still have the same neighbors). For CapD, we took the site that normally auto-cleaves the N terminus and added a FoldIt designed linker between the natural CapD N and C terminus. This circular permuted CapD, CapD_CP, is a monomer which give it greater stability and ease of purification. |
Since the first residue of any nascent protein must be methionine, we rely on E. Coli’s naturally occurring methionine aminopeptidase to remove the first methionine, making CapD_CP catalytically active. The removal of the first methionine has been verified via mass spectrometry. | Since the first residue of any nascent protein must be methionine, we rely on E. Coli’s naturally occurring methionine aminopeptidase to remove the first methionine, making CapD_CP catalytically active. The removal of the first methionine has been verified via mass spectrometry. | ||
Revision as of 23:45, 9 September 2010
We have two main goals when redesigning CapD. First we want to make it stabler and easier to express. Second we want to improve the hydrolytic activity of CapD expediting poly-γ-D-glutamate cleaving, transforming it into a potent anthrax treatment.
Naturally produced CapD auto-cleaves itself to create a dimer. The new N terminus contains a critical catalytic threonine residue which allows the enzyme to cut poly-γ-D-glutamate.
To increase expression and stability, we created a circular permutation of CapD. Typically we view the primary structure of proteins as linear, but when the N and C termini are in close proximity after folding, the protein can be thought of as a circle with a small gap. Frequently, the gap is moved by creating a linker between the naturally occurring N and C termini, then changing the starting and ending residues. This works so long as the amino acid sequence remains the same relative to itself (neighboring amino acids still have the same neighbors). For CapD, we took the site that normally auto-cleaves the N terminus and added a FoldIt designed linker between the natural CapD N and C terminus. This circular permuted CapD, CapD_CP, is a monomer which give it greater stability and ease of purification.
Since the first residue of any nascent protein must be methionine, we rely on E. Coli’s naturally occurring methionine aminopeptidase to remove the first methionine, making CapD_CP catalytically active. The removal of the first methionine has been verified via mass spectrometry.
To increase the hydrolytic ability of CapD_CP, we made point mutations in the active site. We focused our attention on two types of mutations. First, we created point mutations that make hydrogen bonds to a modeled transition state of our substrate in an attempt to stabilize the transition state. Second, we mutated the active site into a more open and polar area in an attempt to increase the ease with which water can enter and participate in a hydrolysis reaction.
To make these point mutations, we used a program called FoldIt to predict how changes in protein structure and composition will affect protein stability. FoldIt provides a 3D representation of a protein's crystal structure which can be manipulated. Manipulation functions include point mutations, insertions, deletions, repacking of side chains, and backbone movement, which FoldIt then assesses for stability. This allows the user to quickly interact with a protein and easily predict how mutations will affect a protein.
 "
"