Team:Newcastle/12 July 2010
From 2010.igem.org
| Line 1: | Line 1: | ||
{{Team:Newcastle/mainbanner}} | {{Team:Newcastle/mainbanner}} | ||
| - | + | =LacI BioBrick Construction= | |
[[Image:P7120450.JPG|300px|thumb|right]] | [[Image:P7120450.JPG|300px|thumb|right]] | ||
| - | + | ==Aims== | |
To use PCR to extract lacI (promoter, ribosome-binding site (RBS) & coding sequence (CDS)) from plasmid pMutin4 and ligate into vector pSB1AT3 in front of red fluorescent protein (RFP). | To use PCR to extract lacI (promoter, ribosome-binding site (RBS) & coding sequence (CDS)) from plasmid pMutin4 and ligate into vector pSB1AT3 in front of red fluorescent protein (RFP). | ||
| Line 11: | Line 11: | ||
# Miniprep PCR - of colonies that worked | # Miniprep PCR - of colonies that worked | ||
| - | + | ==Materials== | |
* PCR reagents | * PCR reagents | ||
| Line 18: | Line 18: | ||
* Wire loop | * Wire loop | ||
| - | + | ==Colony PCR== | |
Of the 11 plates with colonies grown 7 were used for colony PCR and Streak plating. Colony PCR is less accurate than the mini prep however results can be seen quicker whereas the miniprep has a 1 day wait. The colonies selected selected were satellite colonies because ampicillin degraded'''???''' | Of the 11 plates with colonies grown 7 were used for colony PCR and Streak plating. Colony PCR is less accurate than the mini prep however results can be seen quicker whereas the miniprep has a 1 day wait. The colonies selected selected were satellite colonies because ampicillin degraded'''???''' | ||
| Line 45: | Line 45: | ||
'''Note''' Melting temperatures of primers is important and can have a big effect on the product formed (Temperature used see Thursday) | '''Note''' Melting temperatures of primers is important and can have a big effect on the product formed (Temperature used see Thursday) | ||
| - | + | ==Spread Plates== | |
{| | {| | ||
|- | |- | ||
| Line 53: | Line 53: | ||
|} | |} | ||
| - | === | + | ==Overnight culture== |
| + | The seven colonies that grew on the plates were cultured overnight. The [[Team:Newcastle/Growing_an_overnight_cultures| growing an overnight culture]] protocol was followed. | ||
| + | |||
| + | ==Tommorrow== | ||
We are going to take one of the colonies that have worked and possibly a whole plasmid prep. | We are going to take one of the colonies that have worked and possibly a whole plasmid prep. | ||
{{Team:Newcastle/footer}} | {{Team:Newcastle/footer}} | ||
Revision as of 15:08, 6 August 2010

| |||||||||||||
| |||||||||||||
Contents |
LacI BioBrick Construction
Aims
To use PCR to extract lacI (promoter, ribosome-binding site (RBS) & coding sequence (CDS)) from plasmid pMutin4 and ligate into vector pSB1AT3 in front of red fluorescent protein (RFP).
Summary:
- Colony PCR
- Streak plate
- Miniprep PCR - of colonies that worked
Materials
- PCR reagents
- Agar plates
- Gloves
- Wire loop
Colony PCR
Of the 11 plates with colonies grown 7 were used for colony PCR and Streak plating. Colony PCR is less accurate than the mini prep however results can be seen quicker whereas the miniprep has a 1 day wait. The colonies selected selected were satellite colonies because ampicillin degraded???
7 Tubes were labelled 1-7 (+ a control)
The quantities for the PCR are as follows:
- 1 µl Template
- 0.25 µl GoTaq Polymerase
- 2.5 µl forward primer
- 2.5 µl reverse primer
- 1 µl dNTP (10molar stock)
- 10.0 µl 5times GoTaq buffer
- 32.75 µl deionised H20
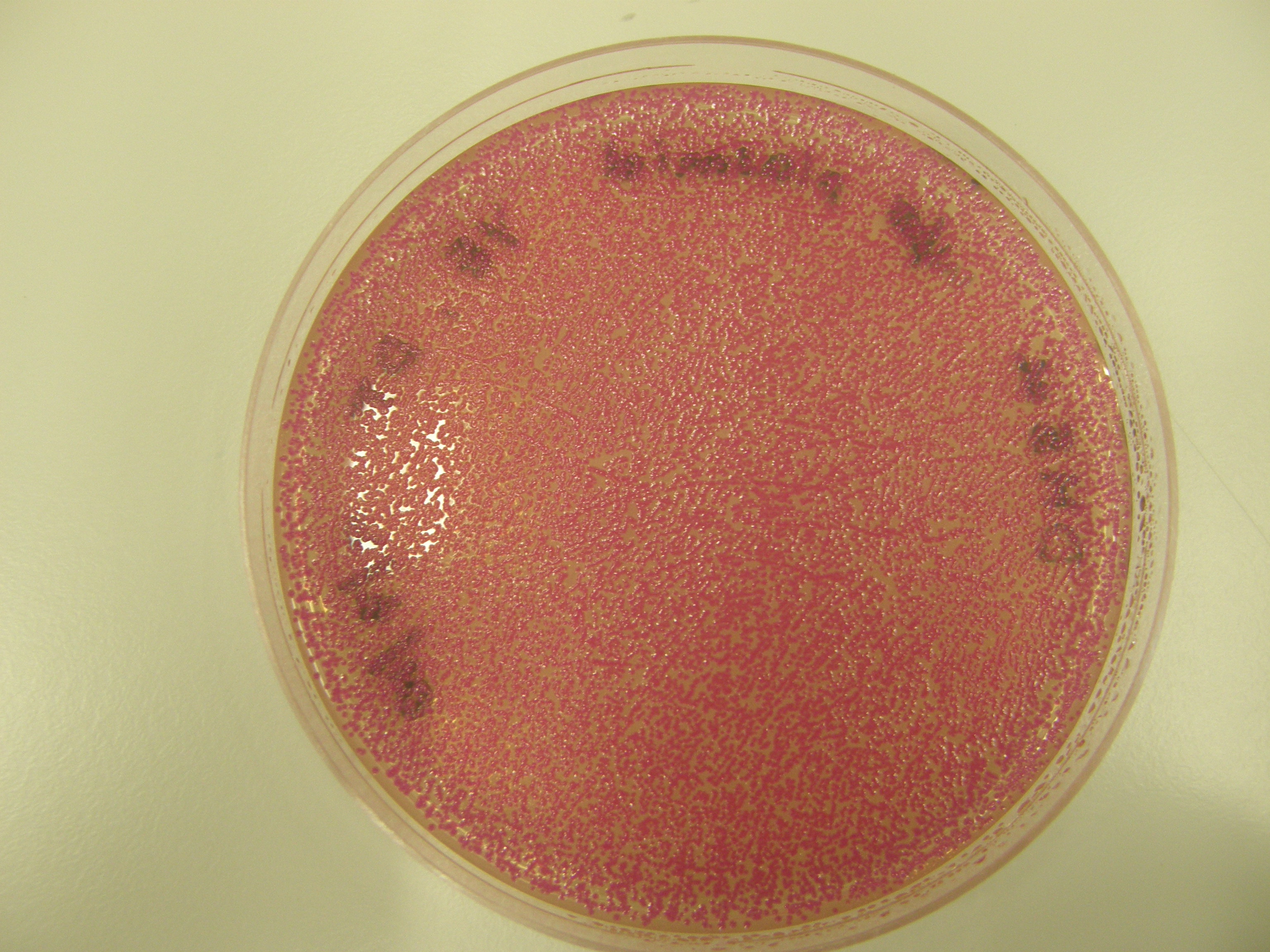
Note There are 2 possible reasons for red colonies formed:
- partial digest - rejoins without insert
- with insert
We need to tell the difference between the two.
Note PCR is best when everything is kept on ice!
Note Melting temperatures of primers is important and can have a big effect on the product formed (Temperature used see Thursday)
Spread Plates
Overnight culture
The seven colonies that grew on the plates were cultured overnight. The growing an overnight culture protocol was followed.
Tommorrow
We are going to take one of the colonies that have worked and possibly a whole plasmid prep.
 
|
 "
"