|
JULY: WEEK 3
July, 12th
Phasins PhaP1 and PhaP2 were sequenced, but none of them has a clear chromatogram, so we decided to sequence the PCR results.
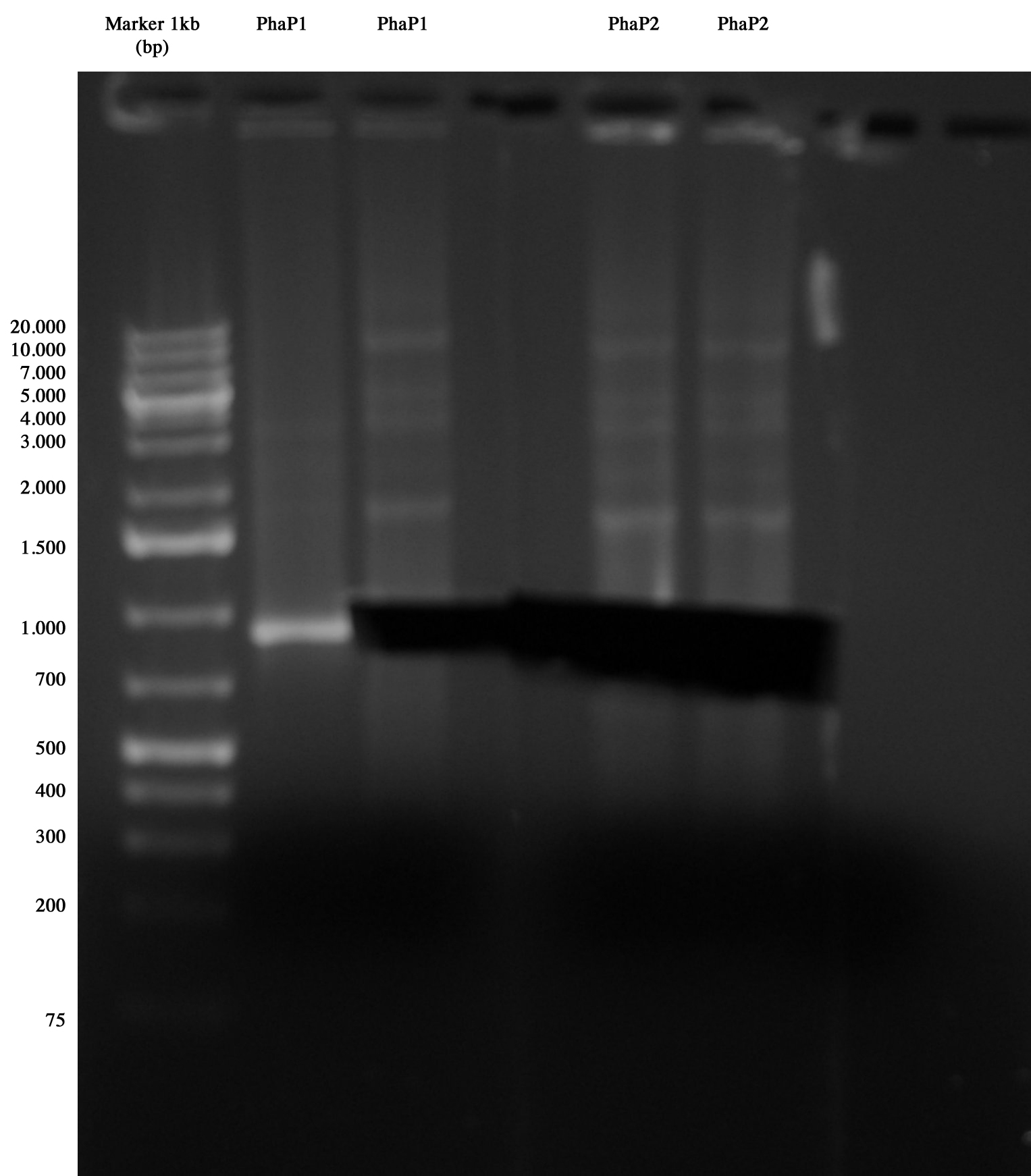 Gel run/cut of phasins amplified via PCR Phasins were gel run/cut and the samples were prepared to be sequenced.
Inoculum of
- <partinfo>BBa_J23101</partinfo>
- <partinfo>BBa_J23105</partinfo>
- <partinfo>BBa_J23106</partinfo>
for tomorrow ligations.
July, 13th
LB+Amp was prepared and phasins sample were sent to be sequenced.
For cultures grown OverNight at 37°C, 220 rpm were MiniPrep was performed:
| <partinfo>BBa_J23101</partinfo> | 96,1 ng/ul
|
| <partinfo>BBa_J23105</partinfo> | 62,8 ng/ul
|
| <partinfo>BBa_J23106</partinfo> | 76,9 ng/ul
|
Other plasmids were retrieved from our freezer:
- <partinfo>BBa_J23118</partinfo> (already digested SpeI-PstI)
- <partinfo>BBa_J23110</partinfo> (already digested SpeI-PstI)
- <partinfo>BBa_J23116</partinfo> (already digested SpeI-PstI)
- I6-2 (already digested XbaI-PstI)
- 4C5 (MiniPrep performed, it will be digested EcoRI-PstI)
- I7-3 (MiniPrep performed, it will be digested EcoRI-PstI)
- I8-5 (MiniPrep performed, it will be digested EcoRI-PstI)
- I10-1 (MiniPrep performed, it will be digested EcoRI-PstI)
- I3-1 (MiniPrep performed, it will be digested XbaI-PstI)
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| 4C5 (x2) | Vector | 25 | 3,6 | 16,9 | 1 EcoRI | 1 PstI | 2,5
|
| I7-3 | Insert | 25 | 14 | 6,5 | 1 EcoRI | 1 PstI | 2,5
|
| I8-5 | Insert | 25 | 12,7 | 7,8 | 1 EcoRI | 1 PstI | 2,5
|
| I10-1 | Insert | 25 | 15,3 | 5,2 | 1 EcoRI | 1 PstI | 2,5
|
| I3-1 (x2) | Vector | 25 | 5,8 | 14,7 | 1 XbaI | 1 PstI | 2,5
|
| <partinfo>BBa_J23105</partinfo> | Insert | 25 | 15,9 | 4,6 | 1SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_J23106</partinfo> | Insert | 25 | 13 | 7,5 | 1 SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_J23101</partinfo> | Insert | 25 | 10,4 | 10,1 | 1 SpeI | 1 PstI | 2,5
|
Digestions were incubated at 37°C for 3hours, then gel run/cut.
Gel was prepared for electrophoresis: 150ml TBE + 1,5 g Agarose + 3ul EtBr.
Ligations were all performed 1:5 (1ul vector + 5ul insert):
- I11= <partinfo>BBa_J23101</partinfo> (S-P) + I6 (X-P)
- I12= <partinfo>BBa_J23105</partinfo> (S-P) + I6 (X-P)
- I13= <partinfo>BBa_J23106</partinfo> (S-P) + I6 (X-P)
- I14= <partinfo>BBa_J23118</partinfo> (S-P) + I3 (X-P)
- I15= <partinfo>BBa_J23110</partinfo> (S-P) + I3 (X-P)
- I16= <partinfo>BBa_J23116</partinfo> (S-P) + I3 (X-P)
- I17= <partinfo>BBa_J23101</partinfo> (S-P) + I3 (X-P)
- I18= <partinfo>BBa_J23105</partinfo> (S-P) + I3 (X-P)
- I19= <partinfo>BBa_J23106</partinfo> (S-P) + I3 (X-P)
- I74C5= I7 (E-P) + 4C5 (E-P)
- I84C5= I8 (E-P) + 4C5 (E-P)
- I104C5= I10 (E-P) + 4C5 (E-P)
PhaP1 and PhaP2 samples were prepared for sequencing.
July, 14th
PBHR68 plate showed colonies!! We picked a colony and inoculated it in LB+Amp. This culture was grown at 37°C 220rpm for 6hours.
Trasformation of ligations in:
| Ligation name | E. coli strain | Resistance |
|---|
- I11= <partinfo>BBa_J23101</partinfo> (S-P) + I6 (X-P)
|
TOP10
|
Amp 100 |
- I12= <partinfo>BBa_J23105</partinfo> (S-P) + I6 (X-P)
|
TOP10
|
Amp 100 |
- I13= <partinfo>BBa_J23106</partinfo> (S-P) + I6 (X-P)
|
TOP10
|
Amp 100 |
- I14= <partinfo>BBa_J23118</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I15= <partinfo>BBa_J23110</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I16= <partinfo>BBa_J23116</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I17= <partinfo>BBa_J23101</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I18= <partinfo>BBa_J23105</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I19= <partinfo>BBa_J23106</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I74C5= I7 (E-P) + 4C5 (E-P)
|
TOP10
|
Cm 12.5 |
- I84C5= I8 (E-P) + 4C5 (E-P)
|
TOP10
|
Cm 12.5 |
- I104C5= I10 (E-P) + 4C5 (E-P)
|
TOP10
|
Cm 12.5 |
PBHR68 plasmid was prepared, grown in LB+Amp and streaked on LB + Amp agar plate.
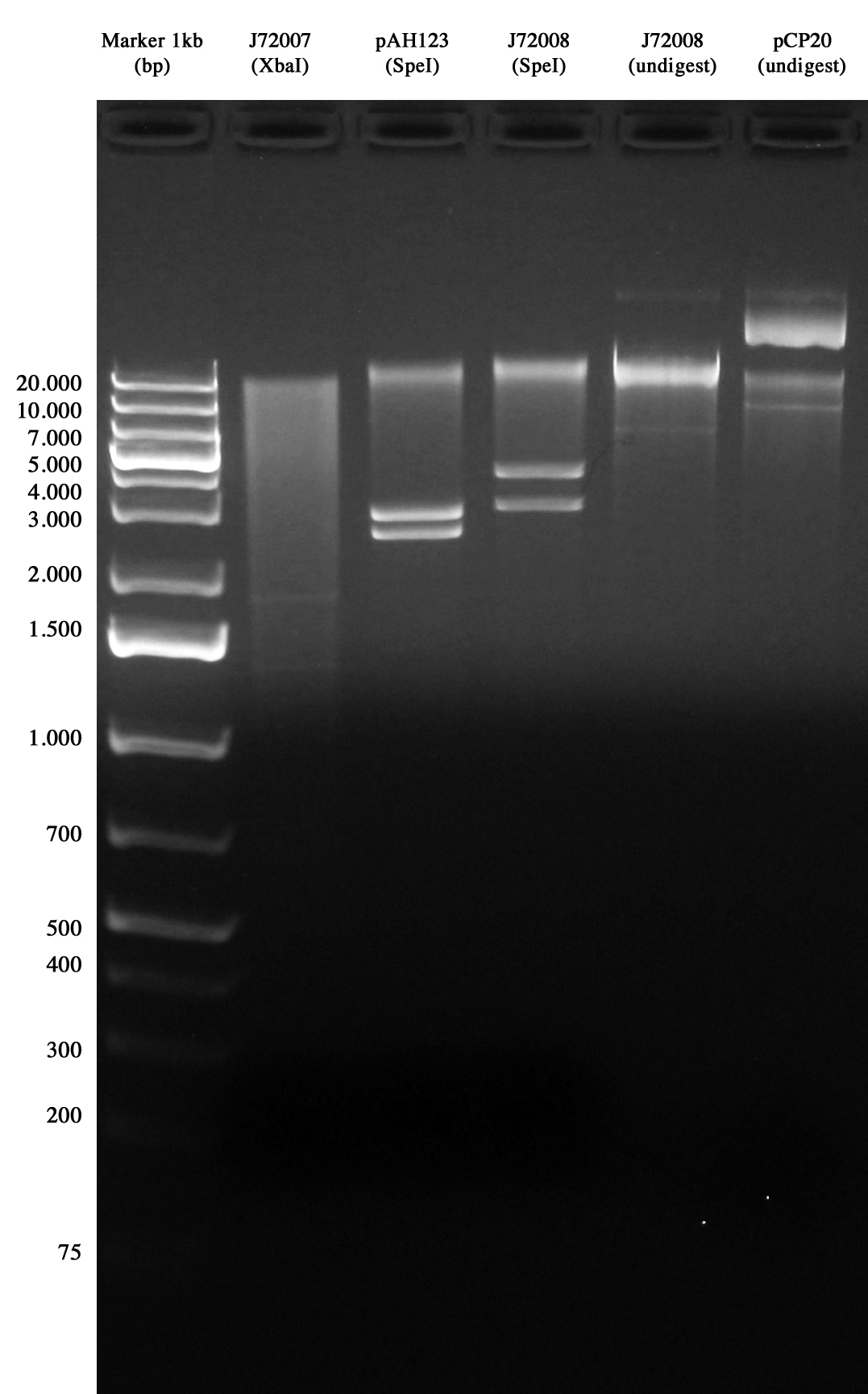 Quality control for HELPER and CRIM plasmids Screening of <partinfo>BBa_J72007</partinfo> (CRIM), <partinfo>BBa_J72008</partinfo> (helper), pAH123 (helper) ans pCP20 (helper). MiniPrep was performed for these cultures (inoculum was performed yesterday).
| <partinfo>BBa_J72007</partinfo> | 45,2 ng/ul
|
| <partinfo>BBa_J72008</partinfo> | 78,0 ng/ul
|
| pAH123 | 81,0 ng/ul
|
| pCP20 | 45,2 ng/ul
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| <partinfo>BBa_J72007</partinfo> | Screening | 25 | 2 | 18,5 | 1 XbaI | --- | 2,5
|
| <partinfo>BBa_J72008</partinfo> | Screening | 25 | 2 | 18,5 | 1 SpeI | --- | 2,5
|
| pAH123 | Screening | 25 | 2 | 18,5 | 1 SpeI | --- | 2,5
|
These samples were digested at 37°C for 1 hour. Gel was loaded with digestions and with <partinfo>BBa_J72008</partinfo> and pCP20 not digested.
Expected length for digestions was:
- <partinfo>BBa_J72007</partinfo>: 1816 and 1351 bp
- <partinfo>BBa_J72008</partinfo>: 3580 and 2755 bp (6335 not digested)
- pAH123: 2755 and 2437
- pCP20: 9400bp
Considered the results obtained, we decided to perform a further screening for <partinfo>BBa_J72007</partinfo>.
Glycerol stock was prepared for PBHR68 and PBHR68 Backup, then falcon tube was re-filled with 5ml LB+Amp for further screening.
Inoculum of <partinfo>BBa_J72007</partinfo> and <partinfo>BBa_J72013</partinfo> were performed in 5ml LB+Cm 34 (HC). Al falcon tubes were placed at 37°C 220 rpm.
Today we received our new enzymes!!!
They were stored at -20°C in "Fermentas Enzymes" box.
July, 15th
We checked agar plates after transformation. All plates were ok, except for:
- I11: only few colonies were observed
- I10-4C5: only 2 colonies were observed
- I15: only few colonies were observed
2 colonies were peaked from every plate and grown in 1ml LB + antibiotic at 37°C, 220rpm for 6 hours. (for colonies grown on LB+Cm, also 1ml LB+Amp was infected)
After that, glycerol stocks were prepared for:
| I11-1 | I11-2 | I12-1 | I12-2
|
| I13-1 | I13-2 | I14-1 | I14-2
|
| I15-1 | I15-2 | I16-1 | I16-2
|
| I17-1 | I17-2 | I18-1 | I18-2
|
| I19-1 | I19-2 | I74C5-1 | I74C5-2
|
| I84C5-1 | I84C5-2 | I104C5-1 |
|
Remaining cultures were re-filled with 5ml LB+abtibiotic and incubated (37°C, 220 rpm) for tomorrow screening.
I10-4C5-2 was grown also in LB+Amp, so it was thrown away!
PBHR68 was grown, ok! :)
MiniPrep was performed for:
- PBHR68 (BioPlastic operon) -> 272 ng/ul
- <partinfo>BBa_J72007</partinfo>(CRIM plasmid) -> 41 ng/ul (not clean spectrum at 230nm)
- <partinfo>BBa_J72013</partinfo>(CRIM plasmid) -> 16 ng/ul
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| PBHR68 | Screening | 25 | 1 | 20,5 | 1 EcoRI | --- | 2,5
|
| <partinfo>BBa_J72007</partinfo> | Screening | 25 | 5 | 16,5 | 1 XbaI | --- | 2,5
|
| <partinfo>BBa_J72013</partinfo> | Screening | 25 | 10 | 10,5 | 1 SpeI | 1 SacI | 2,5
|
Digestions were incubated at 37°C for 3hours, then gel run/cut.
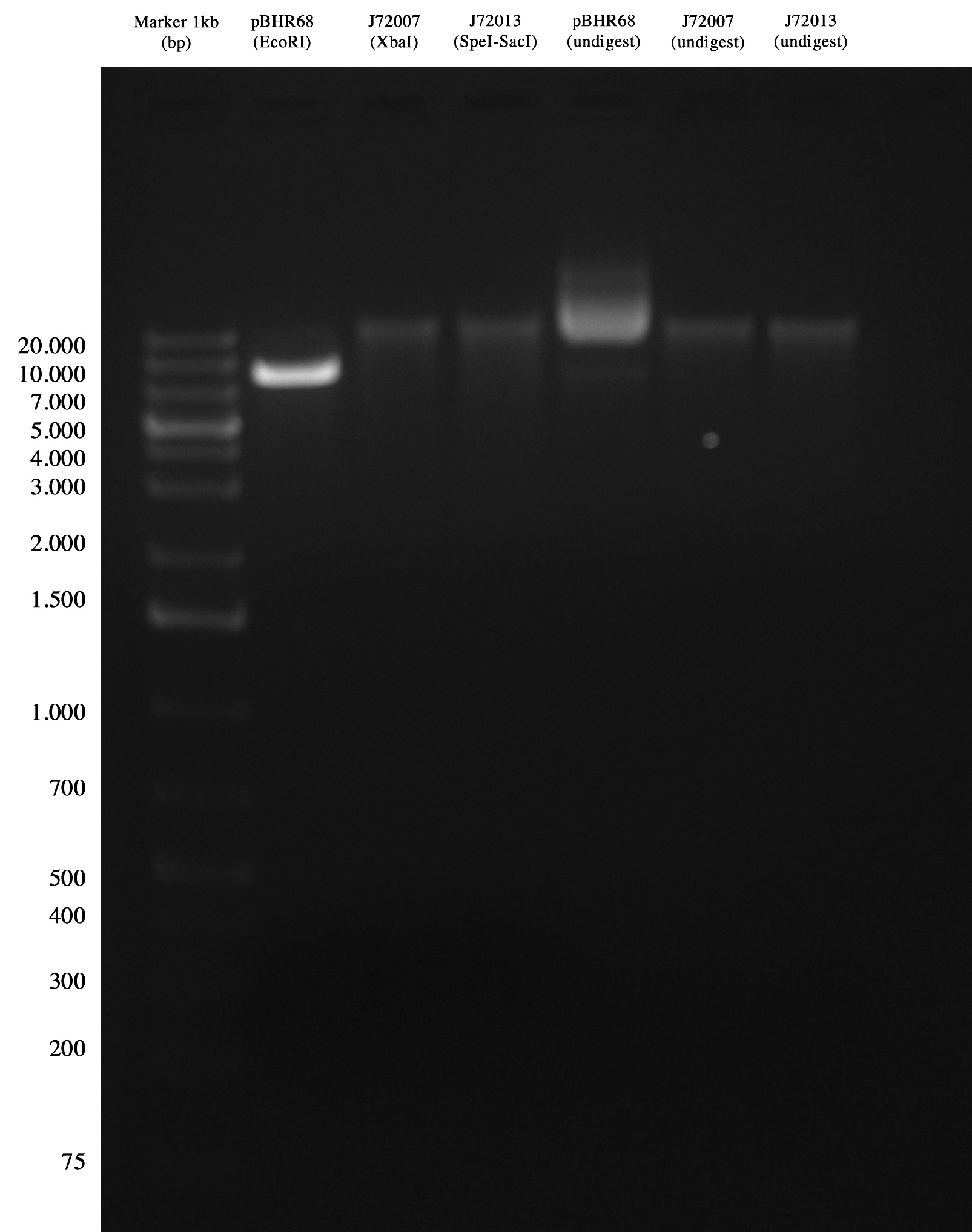 Quality control for pBHR68 and CRIM plasmids Quality control was ok for PBHR68, but no bands were observed for CRIM plasmids.
July, 16th
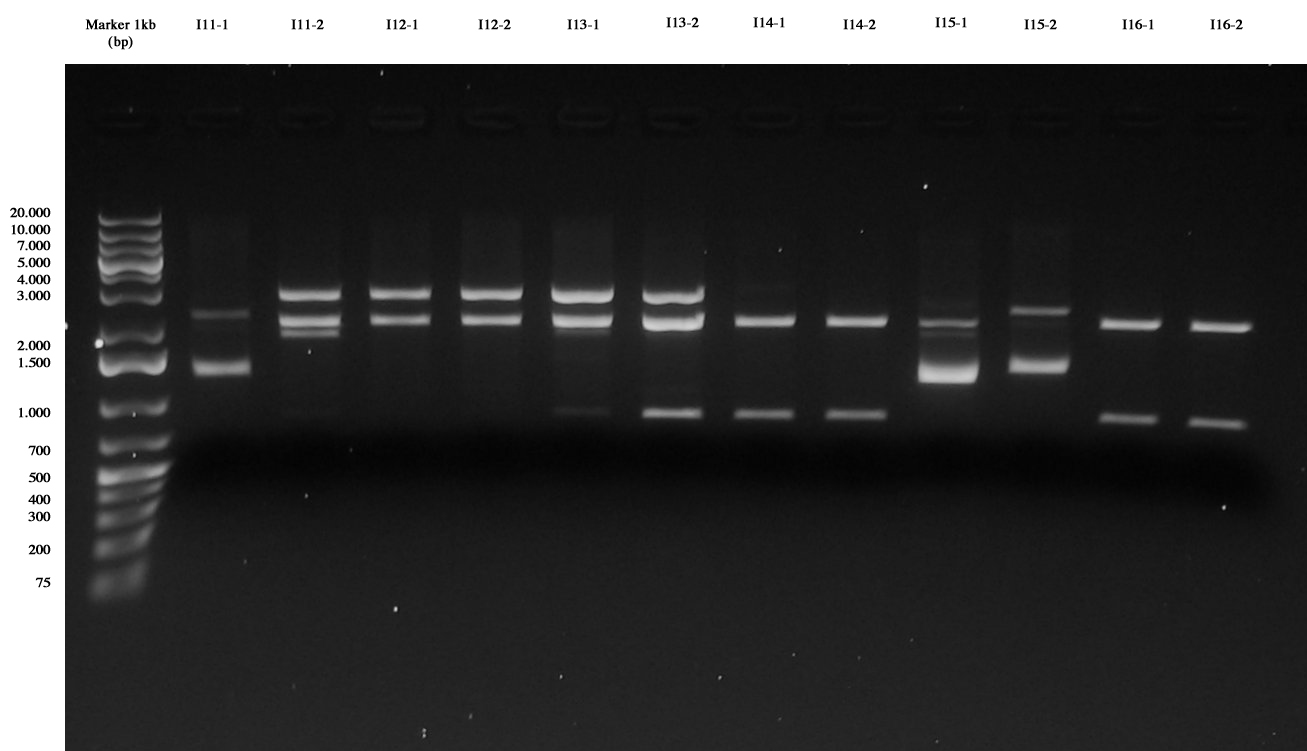 Screening ligations I11-I16 | 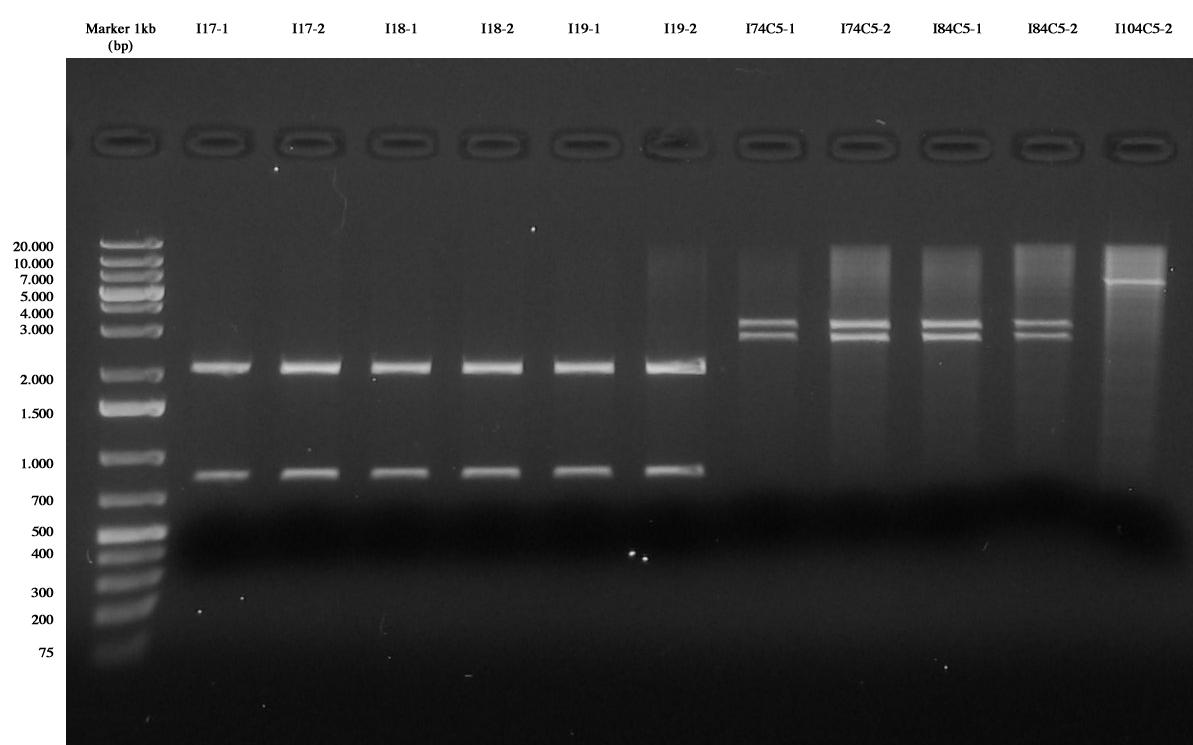 Screening ligations I17-I19, I7/I8/I10-4C5 |
Since it's impossible to isolate the CRIM plasmid <partinfo>BBa_J72007</partinfo> we created a pseudo CRIM plasmid to check that not pir strains aren't able to propagate it.
We called it "THE RING" (RING) and we built it from our I5 (Cm resistance - <partinfo>BBa_P1004</partinfo> - + TT - <partinfo>BBa_B0015</partinfo> - + R6K ori - <partinfo>BBa_J61001</partinfo>) in <partinfo>pSB1A2</partinfo>:
- Digestion of I5 XbaI-PstI
- Gel extraction
- Quantification with Nanodrop (15ng/ul)
- Ligation of X and P sites each other (used 2ul of DNA)
|
|
 "
"





