|
JULY: WEEK 3
July, 12th
Phasins PhaP1 and PhaP2 were sequenced, but none of them has a clear chromatogram, so we decided to amplify them by PCR and sequence results.
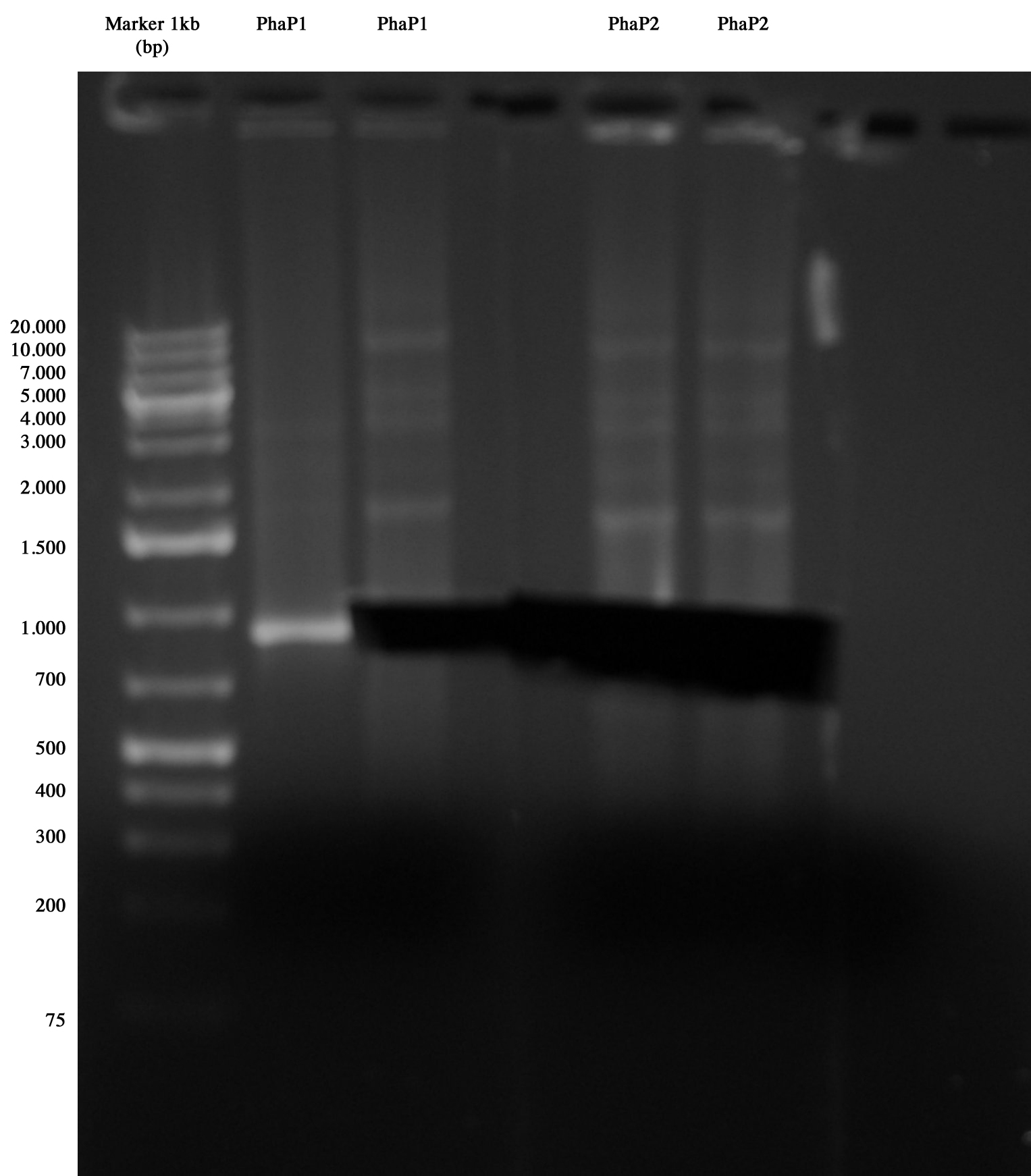 Gel run/cut of phasins amplified via PCR Phasins were gel run/cut and the samples were prepared to be sequenced.
Inoculum of
- <partinfo>BBa_J23101</partinfo>
- <partinfo>BBa_J23105</partinfo>
- <partinfo>BBa_J23106</partinfo>
for tomorrow ligations.
July, 13th
LB+Amp was prepared and phasins samples were sent to be sequenced.
For cultures grown OverNight at 37°C, 220 rpm MiniPrep was performed with the following results:
| <partinfo>BBa_J23101</partinfo> | 96,1 ng/ul
|
| <partinfo>BBa_J23105</partinfo> | 62,8 ng/ul
|
| <partinfo>BBa_J23106</partinfo> | 76,9 ng/ul
|
Other plasmids were retrieved from our freezer:
- <partinfo>BBa_J23118</partinfo> (already digested SpeI-PstI)
- <partinfo>BBa_J23110</partinfo> (already digested SpeI-PstI)
- <partinfo>BBa_J23116</partinfo> (already digested SpeI-PstI)
- I6-2 (already digested XbaI-PstI)
- 4C5 (MiniPrep performed, it will be digested EcoRI-PstI)
- I7-3 (MiniPrep performed, it will be digested EcoRI-PstI)
- I8-5 (MiniPrep performed, it will be digested EcoRI-PstI)
- I10-1 (MiniPrep performed, it will be digested EcoRI-PstI)
- I3-1 (MiniPrep performed, it will be digested XbaI-PstI)
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| 4C5 (x2) | Vector | 25 | 3,6 | 16,9 | 1 EcoRI | 1 PstI | 2,5
|
| I7-3 | Insert | 25 | 14 | 6,5 | 1 EcoRI | 1 PstI | 2,5
|
| I8-5 | Insert | 25 | 12,7 | 7,8 | 1 EcoRI | 1 PstI | 2,5
|
| I10-1 | Insert | 25 | 15,3 | 5,2 | 1 EcoRI | 1 PstI | 2,5
|
| I3-1 (x2) | Vector | 25 | 5,8 | 14,7 | 1 XbaI | 1 PstI | 2,5
|
| <partinfo>BBa_J23105</partinfo> | Insert | 25 | 15,9 | 4,6 | 1SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_J23106</partinfo> | Insert | 25 | 13 | 7,5 | 1 SpeI | 1 PstI | 2,5
|
| <partinfo>BBa_J23101</partinfo> | Insert | 25 | 10,4 | 10,1 | 1 SpeI | 1 PstI | 2,5
|
Digestions were incubated at 37°C for 3 hours, then gel run/cut.
Gel was prepared for electrophoresis: 150ml TBE + 1,5 g Agarose + 3ul EtBr.
Ligations were all performed 1:5 (1ul vector + 5ul insert):
- I11= <partinfo>BBa_J23101</partinfo> (S-P) + I6 (X-P)
- I12= <partinfo>BBa_J23105</partinfo> (S-P) + I6 (X-P)
- I13= <partinfo>BBa_J23106</partinfo> (S-P) + I6 (X-P)
- I14= <partinfo>BBa_J23118</partinfo> (S-P) + I3 (X-P)
- I15= <partinfo>BBa_J23110</partinfo> (S-P) + I3 (X-P)
- I16= <partinfo>BBa_J23116</partinfo> (S-P) + I3 (X-P)
- I17= <partinfo>BBa_J23101</partinfo> (S-P) + I3 (X-P)
- I18= <partinfo>BBa_J23105</partinfo> (S-P) + I3 (X-P)
- I19= <partinfo>BBa_J23106</partinfo> (S-P) + I3 (X-P)
- I74C5= I7 (E-P) + 4C5 (E-P)
- I84C5= I8 (E-P) + 4C5 (E-P)
- I104C5= I10 (E-P) + 4C5 (E-P)
PhaP1 and PhaP2 samples were prepared for sequencing.
July, 14th
PBHR68 plate showed colonies!! We picked a colony and inoculated it in LB+Amp. This culture was grown at 37°C, 220rpm for 6 hours.
Trasformation of ligations in:
| Ligation name | E. coli strain | Resistance |
|---|
- I11= <partinfo>BBa_J23101</partinfo> (S-P) + I6 (X-P)
|
TOP10
|
Amp 100 |
- I12= <partinfo>BBa_J23105</partinfo> (S-P) + I6 (X-P)
|
TOP10
|
Amp 100 |
- I13= <partinfo>BBa_J23106</partinfo> (S-P) + I6 (X-P)
|
TOP10
|
Amp 100 |
- I14= <partinfo>BBa_J23118</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I15= <partinfo>BBa_J23110</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I16= <partinfo>BBa_J23116</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I17= <partinfo>BBa_J23101</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I18= <partinfo>BBa_J23105</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I19= <partinfo>BBa_J23106</partinfo> (S-P) + I3 (X-P)
|
DH5alpha
|
Amp 100 |
- I74C5= I7 (E-P) + 4C5 (E-P)
|
TOP10
|
Cm 12.5 |
- I84C5= I8 (E-P) + 4C5 (E-P)
|
TOP10
|
Cm 12.5 |
- I104C5= I10 (E-P) + 4C5 (E-P)
|
TOP10
|
Cm 12.5 |
PBHR68 plasmid was prepared, grown in LB+Amp and streaked on LB + Amp agar plate.
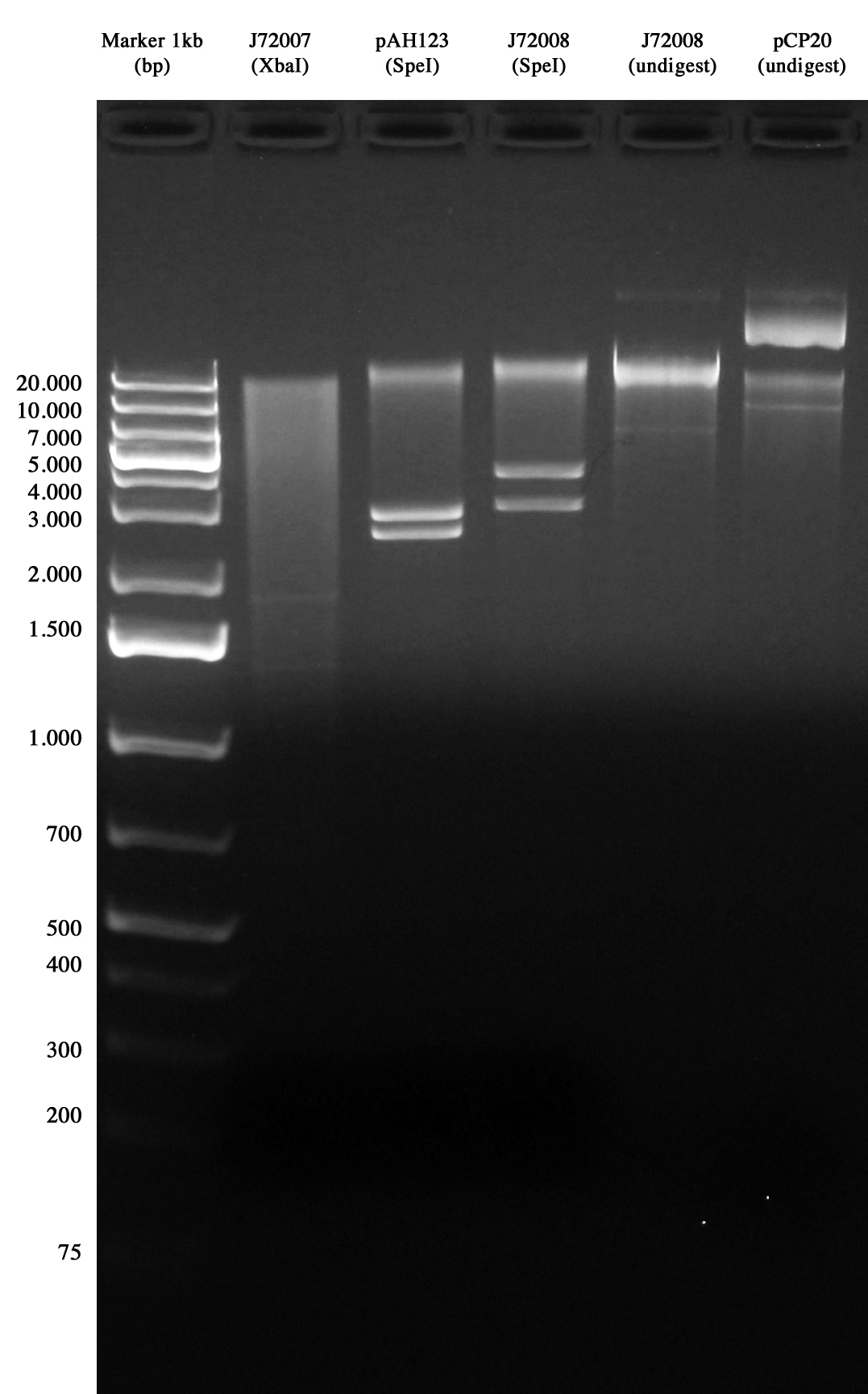 Quality control for HELPER and CRIM plasmids Screening of <partinfo>BBa_J72007</partinfo> (CRIM), <partinfo>BBa_J72008</partinfo> (helper), pAH123 (helper) ans pCP20 (helper). MiniPrep was performed for these cultures (inoculum was performed yesterday).
| <partinfo>BBa_J72007</partinfo> | 45,2 ng/ul
|
| <partinfo>BBa_J72008</partinfo> | 78,0 ng/ul
|
| pAH123 | 81,0 ng/ul
|
| pCP20 | 45,2 ng/ul
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| <partinfo>BBa_J72007</partinfo> | Screening | 25 | 2 | 18,5 | 1 XbaI | --- | 2,5
|
| <partinfo>BBa_J72008</partinfo> | Screening | 25 | 2 | 18,5 | 1 SpeI | --- | 2,5
|
| pAH123 | Screening | 25 | 2 | 18,5 | 1 SpeI | --- | 2,5
|
These samples were digested at 37°C for 1 hour. Gel was loaded with digestions and with <partinfo>BBa_J72008</partinfo> and pCP20 not digested.
Expected length for digestions was:
- <partinfo>BBa_J72007</partinfo>: 1816 and 1351 bp
- <partinfo>BBa_J72008</partinfo>: 3580 and 2755 bp (6335 not digested)
- pAH123: 2755 and 2437
- pCP20: 9400bp
Considered the results obtained, we decided to perform a further screening for <partinfo>BBa_J72007</partinfo>.
Glycerol stock was prepared for PBHR68 and PBHR68 Backup, then falcon tube was re-filled with 5ml LB+Amp for further screening.
Inoculum of <partinfo>BBa_J72007</partinfo> and <partinfo>BBa_J72013</partinfo> were performed in 5ml LB+Cm 34 (HC). All falcon tubes were placed at 37°C 220 rpm.
Today we received our new enzymes!!!
They were stored at -20°C in "Fermentas Enzymes" box.
July, 15th
We checked agar plates after transformation. All plates were ok, except for:
- I11: only few colonies were observed
- I10-4C5: only 2 colonies were observed
- I15: only few colonies were observed
2 colonies were picked from every plate and grown in 1ml LB + antibiotic at 37°C, 220rpm for 6 hours (for colonies grown on LB+Cm, also 1ml LB+Amp was infected).
After that glycerol stocks were prepared for:
| I11-1 | I11-2 | I12-1 | I12-2
|
| I13-1 | I13-2 | I14-1 | I14-2
|
| I15-1 | I15-2 | I16-1 | I16-2
|
| I17-1 | I17-2 | I18-1 | I18-2
|
| I19-1 | I19-2 | I74C5-1 | I74C5-2
|
| I84C5-1 | I84C5-2 | I104C5-1 |
|
Remaining cultures were re-filled with 5ml LB+antibiotic and incubated (37°C, 220 rpm) for tomorrow screening.
I10-4C5-2 was grown also in LB+Amp, so it was thrown away!
PBHR68 was grown, ok! :)
MiniPrep was performed for:
- PBHR68 (BioPlastic operon) -> 272 ng/ul
- <partinfo>BBa_J72007</partinfo>(CRIM plasmid) -> 41 ng/ul (not clean spectrum at 230nm)
- <partinfo>BBa_J72013</partinfo>(CRIM plasmid) -> 16 ng/ul
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| PBHR68 | Screening | 25 | 1 | 20,5 | 1 EcoRI | --- | 2,5
|
| <partinfo>BBa_J72007</partinfo> | Screening | 25 | 5 | 16,5 | 1 XbaI | --- | 2,5
|
| <partinfo>BBa_J72013</partinfo> | Screening | 25 | 10 | 10,5 | 1 SpeI | 1 SacI | 2,5
|
Digestions were incubated at 37°C for 3hours, then gel run/cut.
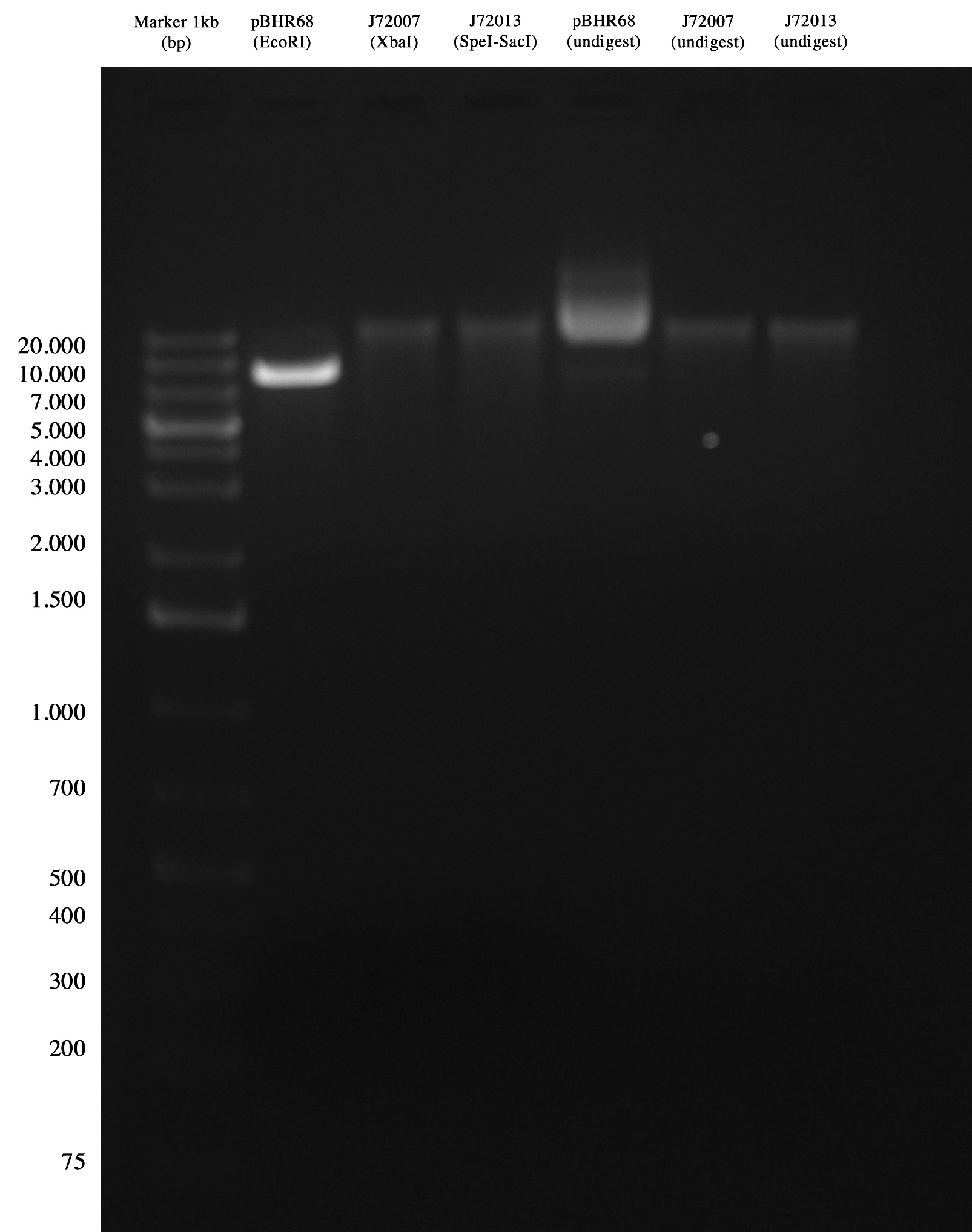 Quality control for pBHR68 and CRIM plasmids Quality control was ok for PBHR68, but no bands were observed for CRIM plasmids.
July, 16th
MiniPrep was performed on 23 falcon tubes containing I11 -> I19 and I7/8/10-4C5 ligations, with the following quantifications:
| I11-1 | 70,2 ng/ul
|
| I11-2 | 99,4 ng/ul
|
| I12-1 | 78,1 ng/ul
|
| I12-2 | 104,3 ng/ul
|
| I13-1 | 152 ng/ul
|
| I13-2 | 168 ng/ul
|
| I14-1 | 71 ng/ul
|
| I14-2 | 70 ng/ul
|
| I15-1 | 42 ng/ul
|
| I15-2 | 103 ng/ul
|
| I16-1 | 68 ng/ul
|
| I16-2 | 58 ng/ul
|
| I17-1 | 55 ng/ul
|
| I17-2 | 89 ng/ul
|
| I18-1 | 65 ng/ul
|
| I18-2 | 58 ng/ul
|
| I19-1 | 58 ng/ul
|
| I19-2 | 70 ng/ul
|
| I7-4C5-1 | 10,9 ng/ul
|
| I7-4C5-2 | 25,9 ng/ul
|
| I8-4C5-1 | 21,1 ng/ul
|
| I8-4C5-2 | 59,5 ng/ul
|
| I10-4C5-2 | 30,2 ng/ul
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer Tango
|
| I11-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I11-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I12-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I12-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I13-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I13-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I14-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I14-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I15-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I15-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I16-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I16-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I17-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I17-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I18-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I18-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I19-1 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I19-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I7-4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I7-4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I8-4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I8-4C5-2 | Screening | 25 | 2 | 19,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I10-4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
Digestions were incubated at 37°C for 3 hours, then gel run.
Gel results showed that:
- I11 ligations are not correct, the same for I13. This is probably due to the fact that promoters for these two parts are too strong, giving an excessive metabolic burden to cells. For this reason, we decided not to repeat these two ligations.
- I12 parts are ok
- I14, I16, I17, I18 and I19 are correct, they will be further screened with a TECAN experiment.
- I15 was wrong, we decided to repeat this ligation, since ligations with stronger promoters were successful.
- I74C5 and I84C5 are ok
- I104C5-2 didn't work. This ligation will be repeated.
We decided to performe a TECAN test on our parts, in order to see if RFP was correctly excided from BBa_J231xx vector, since length of RFP and of our insert (RBS-luxI-tt) were similar. For this reason parts that passed "Gel control" were inoculated:
| I14-1 (LB+Amp) | I14-2 (LB+Amp) | I16-1 (LB+Amp) | I16-2 (LB+Amp) | I17-1 (LB+Amp)
|
| I17-2 (LB+Amp) | I18-1 (LB+Amp) | I18-2 (LB+Amp) | I19-1 (LB+Amp) | I19-2 (LB+Amp)
|
and tomorrow we will measure RFP in cultures, hoping not to find it!! :)
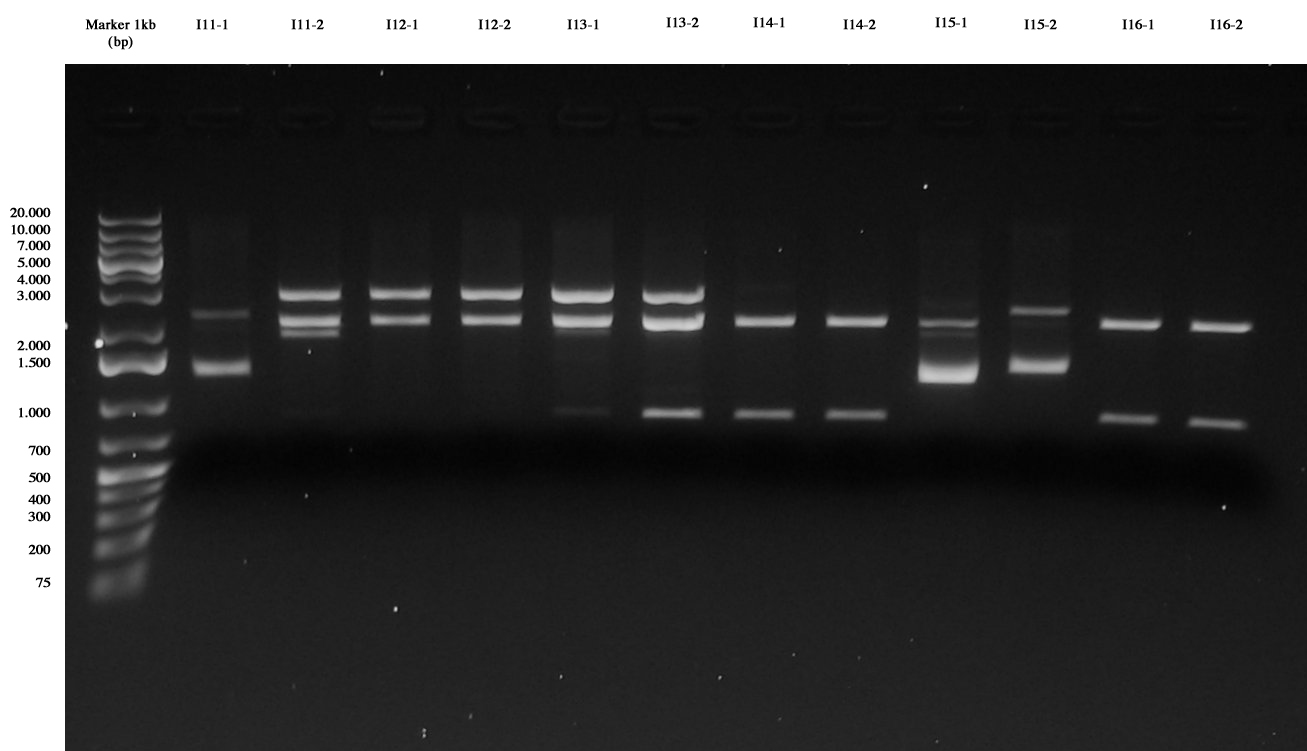 Screening ligations I11-I16 | 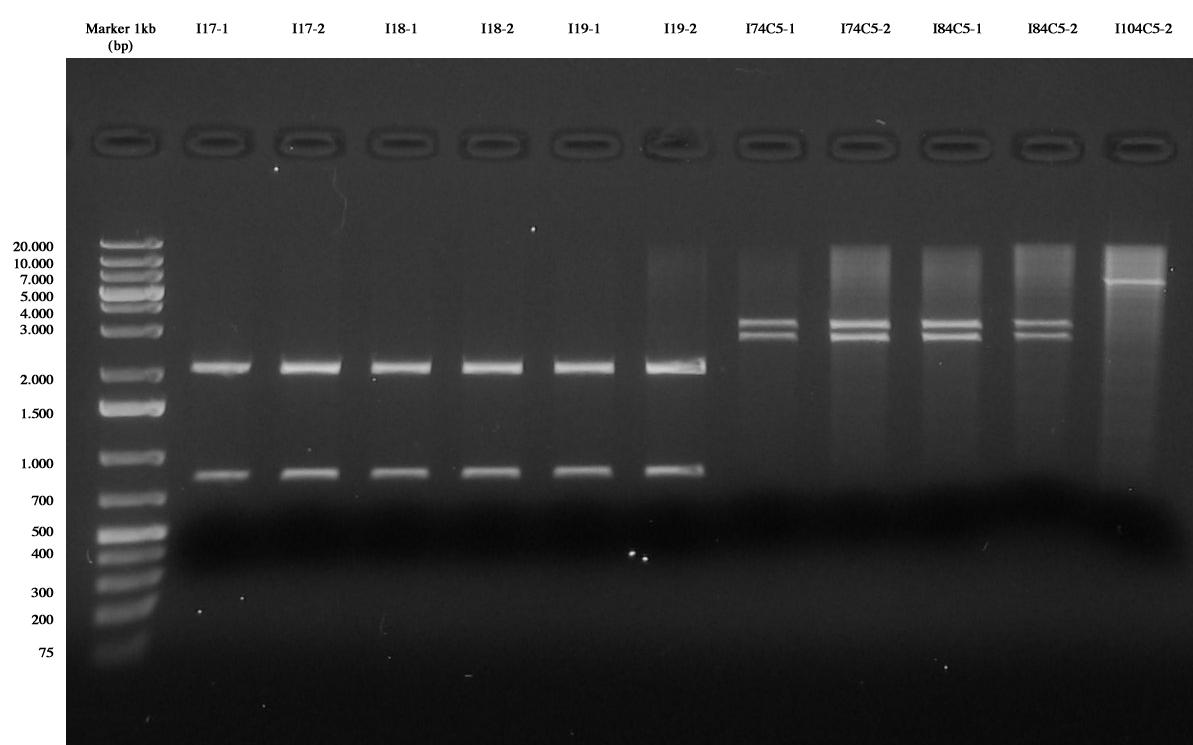 Screening ligations I17-I19, I7/I8/I10-4C5 |
We created a pseudo CRIM plasmid on our own to check that not pir strains aren't able to propagate it.
We called it "THE RING" (RING) and we built it from our I5 (Cm resistance - <partinfo>BBa_P1004</partinfo> - + TT - <partinfo>BBa_B0015</partinfo> - + R6K ori - <partinfo>BBa_J61001</partinfo>) in <partinfo>pSB1A2</partinfo>:
- Digestion of I5 XbaI-SpeI
- Gel extraction
- Quantification with Nanodrop (15ng/ul)
- Ligation of X and S sites each other (used 2ul of DNA)
|
|