Team:UNIPV-Pavia/Calendar/July/settimana3
From 2010.igem.org
(→July, 12th) |
(→July, 13th) |
||
| Line 29: | Line 29: | ||
==July, 13th== | ==July, 13th== | ||
| + | LB+Amp was prepared and phasins sample were sent to be sequenced. | ||
| + | |||
| + | For cultures grown OverNight at 37°C, 220 rpm were MiniPrep was performed: | ||
| + | |||
| + | {| border='1' align='center' | ||
| + | || <partinfo>BBa_J23101</partinfo> || XXX ng/ul | ||
| + | |- | ||
| + | || <partinfo>BBa_J23105</partinfo> || XXX ng/ul | ||
| + | |- | ||
| + | || <partinfo>BBa_J23106</partinfo> || XXX ng/ul | ||
| + | |} | ||
| + | |||
| + | Other plasmids were retrieved from our freezer: | ||
| + | |||
| + | *<partinfo>BBa_J23118</partinfo> (already digested SpeI-PstI) | ||
| + | *<partinfo>BBa_J23110</partinfo> (already digested SpeI-PstI) | ||
| + | *<partinfo>BBa_J23116</partinfo> (already digested SpeI-PstI) | ||
| + | *I6-2 (already digested XbaI-PstI) | ||
| + | *4C5 (MiniPrep performed, it will be digested EcoRI-PstI) | ||
| + | *I7-3 (MiniPrep performed, it will be digested EcoRI-PstI) | ||
| + | *I8-5 (MiniPrep performed, it will be digested EcoRI-PstI) | ||
| + | *I10-1 (MiniPrep performed, it will be digested EcoRI-PstI) | ||
| + | *I3-1 (MiniPrep performed, it will be digested XbaI-PstI) | ||
| + | |||
| + | |||
| + | Digestion of: | ||
| + | |||
| + | {| border="1" align='center' | ||
| + | | ''Culture'' || ''Kind'' || ''Final reaction volume (ul) '' || ''DNA (ul)'' || ''H20 (ul)'' || ''Enzyme 1'' || ''Enzyme 2'' || ''Buffer H'' | ||
| + | |- | ||
| + | | 4C5 (x2) || Vector || 25 || 3,6 || 16,9 || 1 EcoRI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | I7-3 || Insert || 25 || 14 || 6,5 || 1 EcoRI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | I8-5 || Insert || 25 || 12,7 || 7,8 || 1 EcoRI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | I10-1 || Insert || 25 || 15,3 || 5,2 || 1 EcoRI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | I3-1 (x2) || Vector || 25 || 5,8 || 14,7 || 1 XbaI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | <partinfo>BBa_J23105</partinfo> || Insert || 25 || 15,9 || 4,6 || 1SpeI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | <partinfo>BBa_J23106</partinfo> || Insert || 25 || 13 || 7,5 || 1 SpeI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | <partinfo>BBa_J23101</partinfo> || Insert || 25 || 10,4 || 10,1 || 1 SpeI || 1 PstI || 2,5 | ||
| + | |} | ||
| + | |||
| + | Digestions were incubated at 37°C for 3hours, then gel run/cut. | ||
| + | |||
| + | Gel was prepared for electrophoresis: 150ml TBE + 1,5 g Agarose + 3ul EtBr. | ||
| + | |||
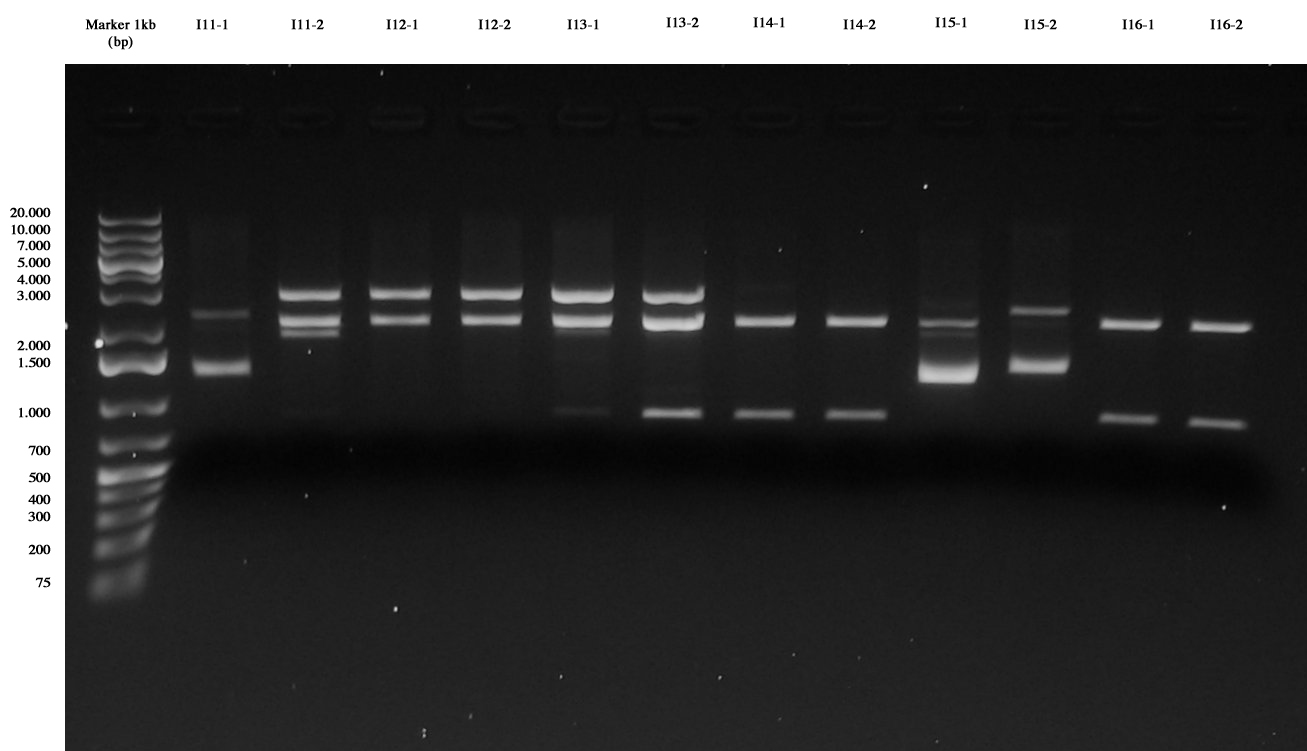
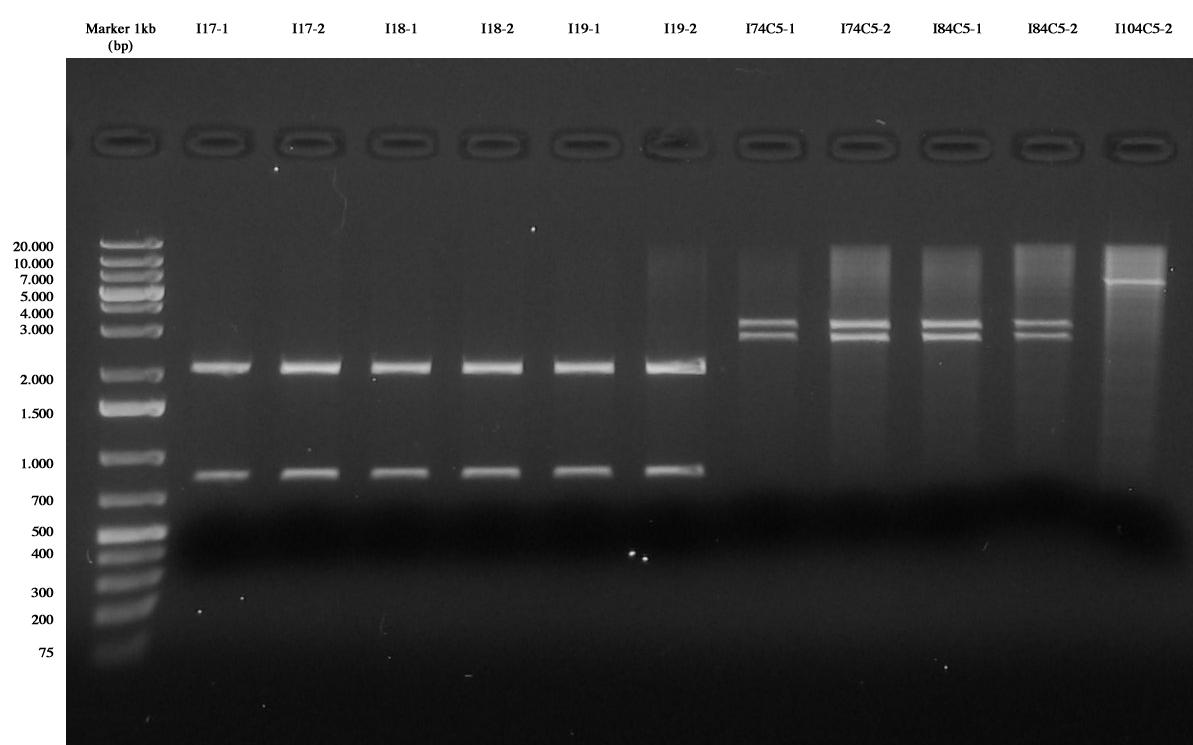
| + | Ligations were all performed 1:5 (1ul vector + 5ul insert): | ||
| + | |||
| + | *I11= <partinfo>BBa_J23101</partinfo> (S-P) + I6 (X-P) | ||
| + | *I12= <partinfo>BBa_J23105</partinfo> (S-P) + I6 (X-P) | ||
| + | *I13= <partinfo>BBa_J23106</partinfo> (S-P) + I6 (X-P) | ||
| + | *I14= <partinfo>BBa_J23118</partinfo> (S-P) + I3 (X-P) | ||
| + | *I15= <partinfo>BBa_J23110</partinfo> (S-P) + I3 (X-P) | ||
| + | *I16= <partinfo>BBa_J23116</partinfo> (S-P) + I3 (X-P) | ||
| + | *I17= <partinfo>BBa_J23101</partinfo> (S-P) + I3 (X-P) | ||
| + | *I18= <partinfo>BBa_J23105</partinfo> (S-P) + I3 (X-P) | ||
| + | *I19= <partinfo>BBa_J23106</partinfo> (S-P) + I3 (X-P) | ||
| + | *I74C5= I7 (E-P) + 4C5 (E-P) | ||
| + | *I84C5= I8 (E-P) + 4C5 (E-P) | ||
| + | *I104C5= I10 (E-P) + 4C5 (E-P) | ||
==July, 14th== | ==July, 14th== | ||
Revision as of 13:45, 26 July 2010
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"