Team:Brown/Notebook/June25
From 2010.igem.org
(→Ligation of ~850 bp amplified insert (denoted WillRS) into pGEM T Easy vector) |
|||
| Line 75: | Line 75: | ||
Used Nanodrop to find DNA concentration (insert) – 49.5 µg/µl? | Used Nanodrop to find DNA concentration (insert) – 49.5 µg/µl? | ||
| - | + | -Blanked with master mix | |
We did not trust the Nanodrop readings because it began giving negative readings. | We did not trust the Nanodrop readings because it began giving negative readings. | ||
Latest revision as of 19:14, 19 July 2010
Friday, June 25 2010
Motility
Took out James’ plates
RESULTS:
| Strain | null plate | amp+IPTG | Chlor+arab |
|---|---|---|---|
| CONTROL | no growth | no growth | no growth |
| DFB 22 | immotile growth | no growth | no growth |
| DFB 201 | immotile growth | no growth | no growth |
| DFB 22 w/ pDB28 | growth | no growth | no growth |
| DFB 201 w/ pDB101 | immotile growth | no growth | motile growth |
| DFB 201 w/ pDB102 | immotile growth | motile growth | no growth |
Bold indicates not expected.
Also null bacteria streaked on BIOL 1220 amp+ plates still grew. This indicates that the amp plates from BIOL1220 are bad.
Also reviewed motility stabs – immotile strains DFB22 and DFB 201 seem immotile, but motile strains are not clear, which is okay as we still have 24 to 36 of incubation time to go.
It seems as though DFB 22 w/ pDB28 has consistent no growth, and so that strain is possibly nonviable (which is okay, as DFB is motB, not motAmotB, which is more interesting).
In general, it appears as though the stabs were more effective than the plates in inducing growth.
Miraculin
Lactobacillus acidophilus aliquoting
NCFM and 43121
43121 Each aliquot has 500 µl dH2O and 200 µl bacteria (+H2O)
NCFM “”
Plates:
- Null plates – 200 µl stock
- Chlor plate – 100 µl each
PCR of pWill1
- 2 µl forward primer
- 2 µl reverse primer
- 1 µl plasmid
- 25 µl master mix
- 20 µl dH2O
50 µl total volume
PCR program on thermocycler: “IGEM”
Autoclaved LB + agar to make more chloramphenicol plates. Chloramphenicol at 15 µg/ml; made plates.
Gave Gary pWill1 and pTG262
Ligation of ~850 bp amplified insert (denoted WillRS) into pGEM T Easy vector
pGEM is already linearized.
Used Nanodrop to find DNA concentration (insert) – 49.5 µg/µl? -Blanked with master mix
We did not trust the Nanodrop readings because it began giving negative readings. So, we are unsure of the concentration and don’t want to do the ligation just yet.
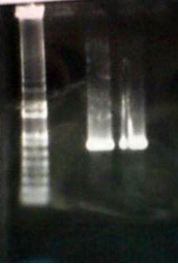
Ran gel (1% Agarose) of PCR product
- Lane 1 – 1kb+ ladder (~20µl)
- Lane 2 – empty
- Lane 3 – Lily PCR (20µl)
- Lane 4 – Ethan PCR (20µl)
QIAGEN gel extraction
- Cut out 2 bands (~850 bp, expected)
- Weight: 0.19 g
- Added 570 µl Buffer QG, followed through with protocol and eluted 50 µl buffer EB. (2 tubes, 100 µl total)
Ligation
- 1 µl 10X T4 DNA ligase buffer
- 1 µl pGEM T Easy (~3kb)
- 3 µl insert (~850 bp)
- 4.5 µl dH2O
- 0.5 T4 DNA Ligase
10 µl total volume
RT for 0.5 hr Then 65°C to denature ligase
Product put into WRD freezer box.
Control ligations:
- No insert; dH2O instead
- No vector; dH2O instead
 "
"