Team:MIT mammalian Switch
From 2010.igem.org
| Line 27: | Line 27: | ||
<li><a href="https://2010.igem.org/Team:MIT_tmodel">Modelling</a></li> | <li><a href="https://2010.igem.org/Team:MIT_tmodel">Modelling</a></li> | ||
<li><a href="https://2010.igem.org/Team:MIT_tconst">Toggle Construction</a></li> | <li><a href="https://2010.igem.org/Team:MIT_tconst">Toggle Construction</a></li> | ||
| - | <li><a href="https://2010.igem.org/Team: | + | <li><a href="https://2010.igem.org/Team:MIT_composite">Characterization</a></li> |
</ul> | </ul> | ||
</dd> | </dd> | ||
Revision as of 01:10, 28 October 2010
|
Synthetic Switch: Bistable Toggles in Mammalian Cells |
| WHY SYNTHETIC SWITCHES?
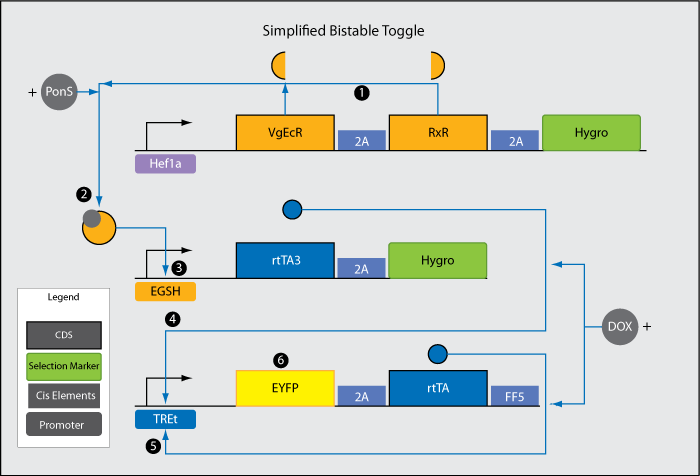
It is useful for a synthetic system capable of sensing mechanical stimuli to be able to convert a transient signal into a constitutive response. We want our system to sense mechanical stimuli for a period of time, during which an internal switch is flipped from the "off" state to the "on" state. After the removal of the stimulus, the cell will stay in the "on" state, during which it secretes BMP2 and osteogenesis occurs. Because cell differentiation occurs on a much longer time scale than desired mechanical stimulation, such a switch is required for a system to be of use. HOW POSITIVE FEEDBACK CAN BE USED TO CONSTRUCT A BISTABLE TOGGLE Consider a transcriptional positive feedback system, in which an outside signal causes the transcription of an inducer of transcription, protein A, which induces the transcription and production of more protein A. When the signal is added, transcription and translation of protein A increases, which causes its own transcription to increase further. The outside stimulating signal reaches a threshold value such that the removal of the signal does not affect the level of protein A production, because at this point almost all the transcription of protein A is driven by protein A, and not the outside signal. At this point we say the cell has reached a new high output state. This state is stable down to a different threshold value of protein A than the previous threshold. Thus, using positive feedback loops, we can construct a bistable toggle with two stable states: low transcription, and high transcription. SIMPLIFIED BISTABLE TOGGLE During the summer we designed both a complete and a simplified bistable toggle with a low and high output state using positive feedback loops.
1. Hef1a promoter leads to low level, constitutive expression of the two halves (VgEcR and RxR) of a receptor responsive to the chemical ponasterone A (PonS). 2. VgEcR, RxR, and PonS combine to form an active complex. 3. EGSH is induced by the VgECR-RxR-PonS complex. 4. rtTA3 is transcribed after teh activation of EGSH. 5. Each rtTA3 binds to two of the six tetO sites in the TREt promoter and induces the promoter. 6. rtTA, another version of rtTA3, further induces activation of the TREt promoter. This establishes a positive feedback loop, which propels the system from a low to a stable high output state (the switch is "flipped"). In the high output state, EYFP is constitutively expressed at a high level. Results The figure below show a preliminary experiment during which PonS was added post transient calcium phosphate transfection of a HEK293FT cell line containing the Hef1a_VgEcR_RxR construct with the other two components of the toggle. |

There is significant increase in fluorescence in the cells transfected with the toggle within 7 hours after the addition of ponS. We did not have enough DNA to perform a parallel transfection without PonS addition; however, we argue that the increase in fluorescence we saw 7 hours after PonS addition is much greater than in the previous 41 hours post co-transfection. Thus it can be concluded that the co-tranfescted construct shows EYFP fluorescence with, but not without, PonS induction. |
FULL MAMMALIAN TOGGLE We also designed a full bistable toggle designed to drastically shorten the off-->on and on-->off switch via microRNA. Arvind, a member of the iGEM team, has mathematically modeled this circuit in depth, along with several others that were also considered. Please view the model here. |
 "
"
 iGEM 2010
iGEM 2010