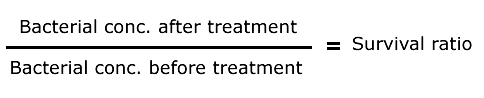Team:Valencia/lea
From 2010.igem.org
Alejovigno (Talk | contribs) (→Results) |
(→Introduction) |
||
| Line 9: | Line 9: | ||
==Introduction== | ==Introduction== | ||
This part of the project involves dealing with harsh temperature changes of the Martian surface. To do so, we implement the expression of LEA (late embryogenesis abundant) “antifreeze” protein. | This part of the project involves dealing with harsh temperature changes of the Martian surface. To do so, we implement the expression of LEA (late embryogenesis abundant) “antifreeze” protein. | ||
| - | Thus, we aim to verify the resistance to cold shock of the E. coli cultures who which express LEA and compare this result with the ones | + | Thus, we aim to verify the resistance to cold shock of the E. coli cultures who which express LEA and compare this result with the ones which doesn't (control cultures). |
Revision as of 00:05, 28 October 2010
Time goes by...
(El tiempo pasa...)
Follow us:

Our main sponsors:

Our institutions:

Visitor location:
» Home » The Project
Surviving on Mars
Introduction
This part of the project involves dealing with harsh temperature changes of the Martian surface. To do so, we implement the expression of LEA (late embryogenesis abundant) “antifreeze” protein. Thus, we aim to verify the resistance to cold shock of the E. coli cultures who which express LEA and compare this result with the ones which doesn't (control cultures).
Late Embryogenesis Abundant (LEA) proteins
Characteristics and function
The study of natural desiccation experienced by embryos during seed maturation allowed the discovery of a large group of genes that encode proteins called LEA (Late embryogenesis abundant). These genes are regulated by osmotic stress and the LEA proteins that they encode are suspected of being involved in the protection of cells to desiccation (Taiz and Zeiger, 1991).
The LEA (Late Embryogenesis Abundant) proteins were discovered about thirty years ago and were identified as proteins that highly accumulate during the maturation of cotton embryogenesis (Dure and Chlan, 1981). Between their common characteristics, we can point out their high hydrophilicity, the abundance of some amino acid residues such as Gly, Ala, Thr, Glu and Lys/Arg, and their probable intrinsic lack of tertiary structure (Battaglia et al. ,2008).
Although these proteins are known for their role in preventing dehydration in plants, they can be found in many distant branches of the tree of life, like in algae, animals, fungi, archaea and bacteria (Tompa and Kovacs, 2010).
Although the action way of LEA proteins is not well understood, is known to accumulate in vegetative tissues during episodes of osmotic stress. Its protective role may be associated with the capacity to hold water (due to their high hydrophilicity) and prevent aggregation of cellular proteins and crystallization of other molecules during drying (Taiz and Zeiger, 1991). They could also help stabilize the cell membrane.
LEA proteins as extreme temperatures protector
Plants suffer water stress, not only during drought or high salt concentrations but also during low temperature conditions. During exposure of plants to freezing temperatures extracellular ice formation imposes a dehydrator force to unfrozen intracellular solution. Ice formation leads to rapid extracellular concentration of solutes and the change of chemical potential and osmotic pressure causes the liquid water moves out of the cell. It’s immediate to deduce that high temperatures also cause water stress and desiccation (Puebla and Del Viso, 2004)
Thus, if the stress caused by low and high temperatures is associated with water stress, LEA proteins could be able to protect cells against extreme temperature. This is where it makes sense the use of LEA proteins in our project. We use them in order to protect the cells would have to survive on Mars, at extremely low temperatures, and should withstand repeated freezing and thawing (depending on the temperature differences between day and night and different seasons).
In this case, soybean PM2 protein, one LEA3 protein (LEA proteins are classified in seven groups according the structure of amino acid sequence), has been chosen. Based on an article (Liu, et al. 2009), we proposed using this protein as a cell protector, and we designed a series of experiments to verify the resistance to temperature changes in E.coli expressing LEA.
Verification of LEA protection against extreme temperature
Extreme temperatures
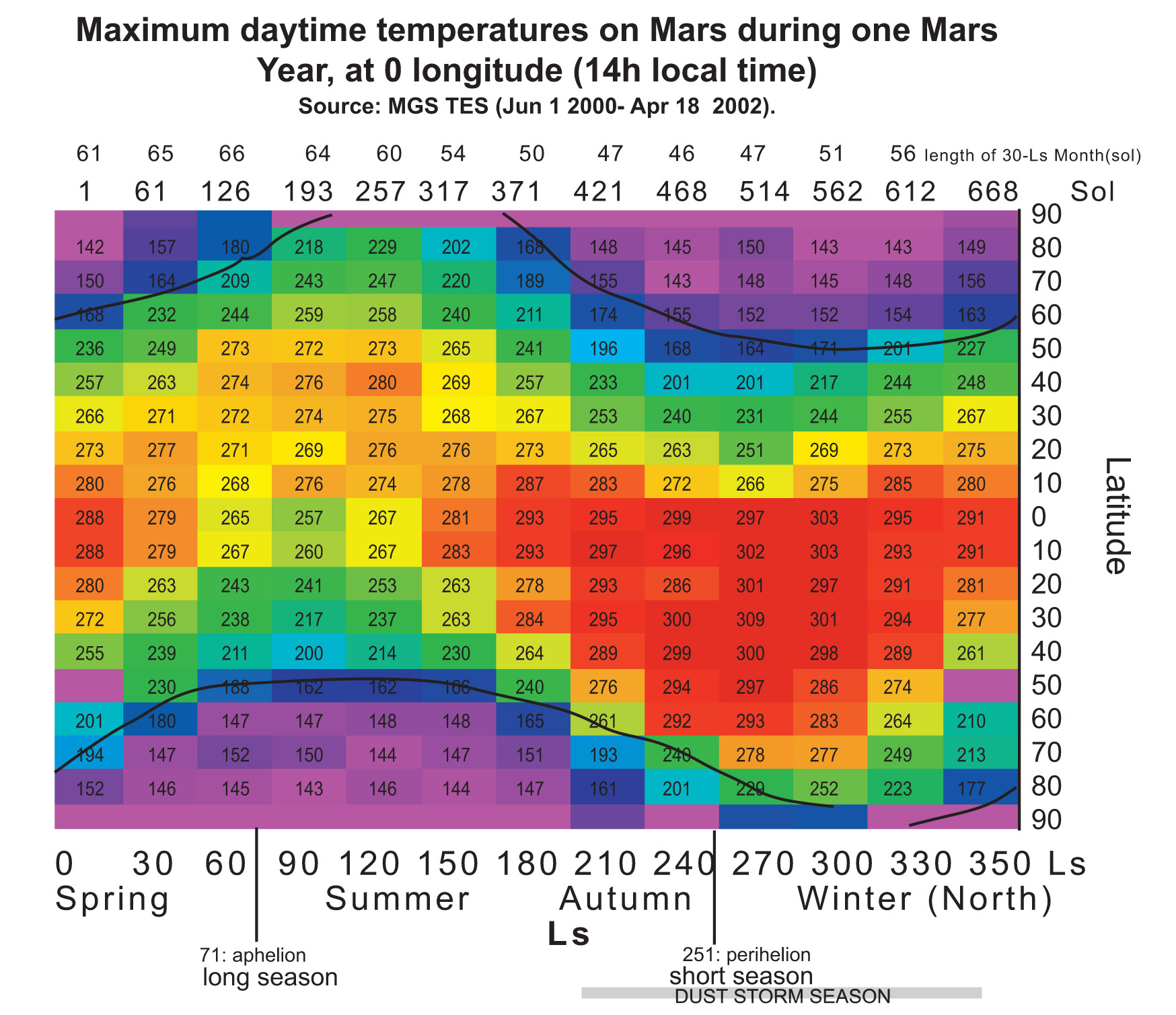
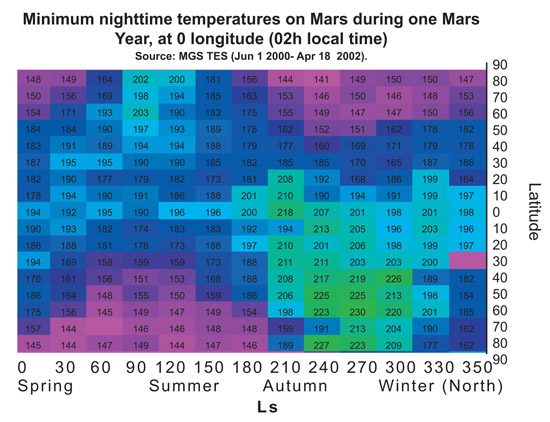
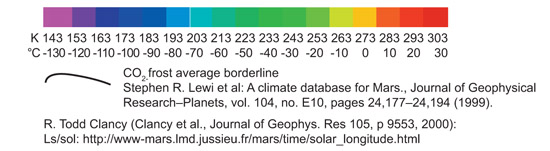
In order to demonstrate that PM2 (LEA3) protein is useful to protect the cells during a part of terraforming process we tried to choose representative temperatures for the treatments. The chart below shows the maximum and minimum temperatures on Mars along a year, depending on the latitude at 0 longitude. Treatment temperatures were selected focusing on the equator area (around latitude 0). In this area current temperatures range, during most of the year, are between -80ºC and 20ºC approximately. And, in a partially terraformed Mars, these temperatures would be warmer.
The considered temperatures are as follows:
- -80ºC, as the minimum temperature on the equator area,
- 20ºC, as the maximum temperature on the equator area,
- -20ºC, as the minimum temperature on the equator area in a partially terraformed Mars,
- 50ºC, as the upper limit of temperature on Mars during the terraforming process.
These experiment temperatures are realistic for the equator area. In colder areas we can use the Red-House device to reach them. Thus, if we demonstrate the cell resistance at these temperatures we might consider these cells be able to live in other areas (and also to grow) because of the temperature rise achieved with the Red-House.
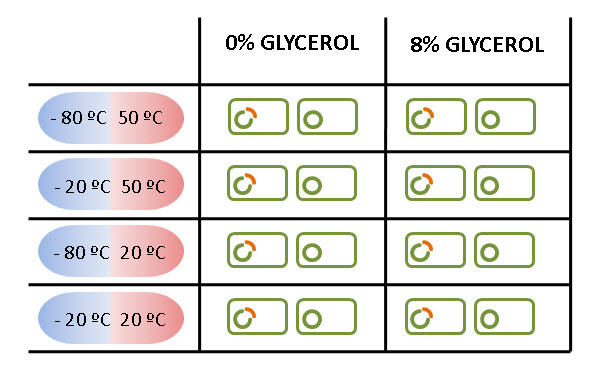
Moreover, in order to mimic the cycle temperatures during a solar day on Mars (sol), the treatments for the experiments consists of varying the temperature from maximum value to a minimum. So, the result of combining these temperatures are four different treatments.
In addition, we also carried on this assays with an 8% of glycerol as assistance for survival that could enhance the effects of LEA protein.
Verification protocol
The experiments was carried on both in E. coli expressing LEA and in others transformed only with the backbone. Later, both results will be compared to demonstrate that LEA really helps E. coli to survive.
The figure below summarizes all the treatments carried on in LEA and NOT LEA cells.
Furthermore, we repeated, for each case, three times in order to obtain three independent events for our results.
Liquid medium was chosen to the treatments, and, after that, the cells was spread in plates to calculate the concentration.
You can see the whole protocol at the Protocols section.
Results
The measured output is the survival ratio calculated as follows:
Bacterial concentration has been calculated from number of colonies counted on the plates before and after the treatment (taking into account initial DO, glycerol concentration and dilutions).
Obtained data has been analyzed by means of a non parametric Kruskall-Wallis analysis. This analysis compares survival ratio obtained in the different conditions: with and without LEA, and with and without glycerol. The confidence value has been set at 0,05.
An independent analysis has been subject for each combination of conditions.
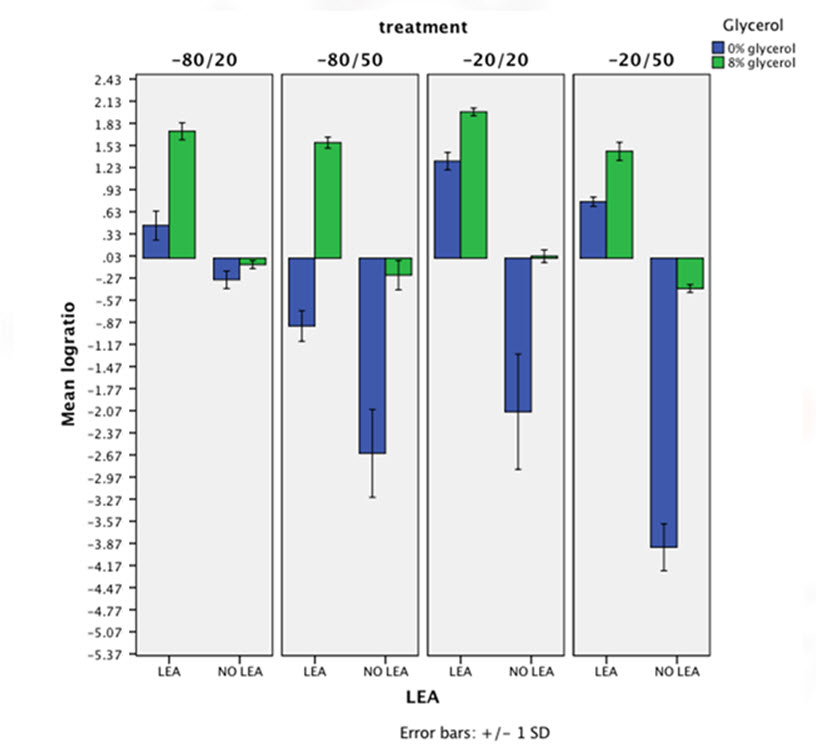
Chart below represents in logarithm axes the mean of survival ratio for all different conditions. This chart clearly shows that expressing LEA cells survive (and grow) while non expressing LEA cells die. So, protective effect of LEA is visible in this figure.
For all analysis obtained p-value is less than 10-4. This points the protective effect of LEA as statically significant.
Finally, watching the chart a synergic interaction between glycerol an LEA is detected. When both LEA protein being expressed and glycerol present in the growth medium the survival ratio combines the two beneficial effects resulting in a much bigger protective effect.
References
Liu, Y., Zheng, Y., Zhang, Y., Wang, W., Li, R. (2009), Soybean PM2 protein (LEA3) confers the tolerance of Escherichia coli and stabilization of enzyme activity under diverse stresses, Current Microbiology, 60: 373-378
Dure, L., Chlan, C. (1981), Developmental Biochemistry of Cottonseed Embryogenesis and Germination, Plant Physiol. 68: 180-186
Battaglia, M., Olvera-Carrillo, Y., Garciarrubio, A., Campos, F., Covarrubias A. (2008), The Enigmatic LEA Proteins and Other Hydrophilins, Plant Physiol, 148: 6-24
Tompa, P., Kovacs, D. (2010), Intrinsically disordered chaperones in plants and animals, Biochemical and Cellular Biology, 88: 167-174
Puebla, A. F., Del Viso, F. (2004), Biotecnología y mejoramiento vegetal, Ediciones INTA, Buenos Aires Taiz, L., Zeiger, E. (1991). Plant Physiology, Benjamin/Cummings Pub. Co., New York
 "
"