Team:Heidelberg/Project/Mouse Infection
From 2010.igem.org
(→construct schemes) |
(→in vivo study) |
||
| Line 46: | Line 46: | ||
==Results== | ==Results== | ||
| - | === | + | ===Constructs=== |
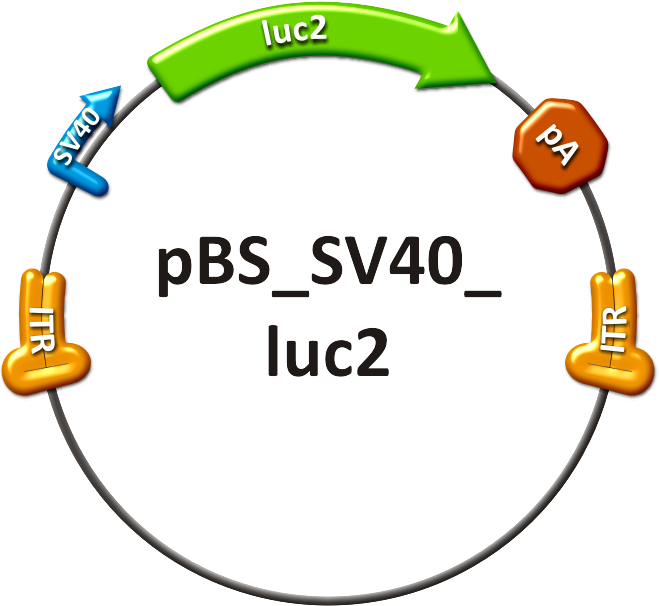
The <i>in vivo</i> analysis should enlighten our gene therapy approach using AAV tropism as well as miRNA binding sites as trigger for expression. The following constructs have been subcloned separately into the AAV context to accomplish those tasks: <br/> | The <i>in vivo</i> analysis should enlighten our gene therapy approach using AAV tropism as well as miRNA binding sites as trigger for expression. The following constructs have been subcloned separately into the AAV context to accomplish those tasks: <br/> | ||
# positive control, | # positive control, | ||
| Line 60: | Line 60: | ||
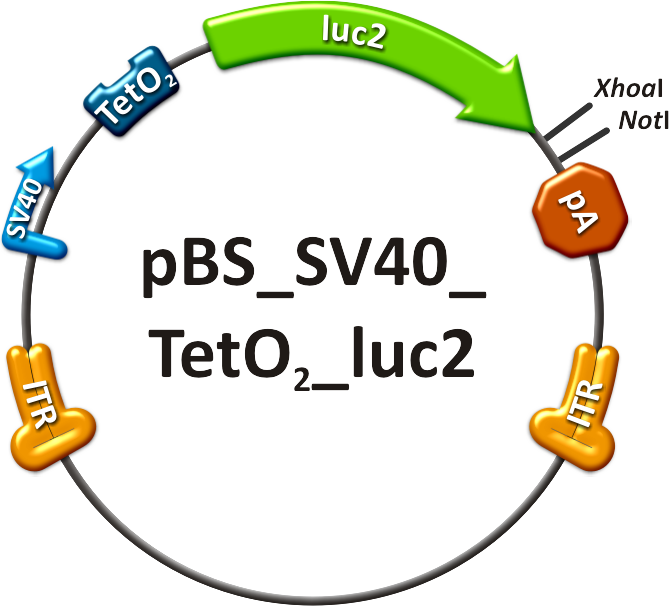
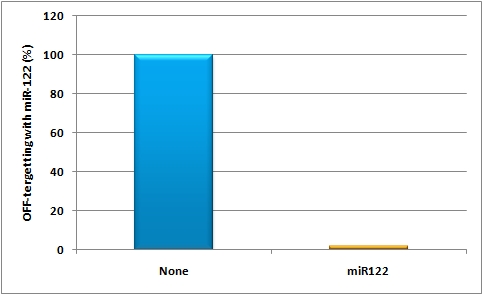
# The on-targeting construct consisted of two independent viruses which were co-infected into mice, as well. One of these viruses packaged the Tet Repressor (TetR) driven by an SV40 promoter ("repressor" construct, see sidebar, fig. 5). The expression of TetR is under the control of miR-122 as four binding sites of this miRNA were cloned into the 3’UTR of the gene. The second virus was composed of an SV40 promoter driving the Tet operator (TetO<sub>2</sub>) which monitors the expression of luc2 ("operator" construct, see sidebar, fig. 6). With this setup, luc2 expression should be inhibited by the TetR in all mice tissues except for liver cells, where TetR is down-regulated by miRNA 122. | # The on-targeting construct consisted of two independent viruses which were co-infected into mice, as well. One of these viruses packaged the Tet Repressor (TetR) driven by an SV40 promoter ("repressor" construct, see sidebar, fig. 5). The expression of TetR is under the control of miR-122 as four binding sites of this miRNA were cloned into the 3’UTR of the gene. The second virus was composed of an SV40 promoter driving the Tet operator (TetO<sub>2</sub>) which monitors the expression of luc2 ("operator" construct, see sidebar, fig. 6). With this setup, luc2 expression should be inhibited by the TetR in all mice tissues except for liver cells, where TetR is down-regulated by miRNA 122. | ||
| - | === | + | ===Mice injection=== |
The tail vein injection was chosen so as to assess AAV serotype tissue tropism; | The tail vein injection was chosen so as to assess AAV serotype tissue tropism; | ||
the luciferase transgene was used for visualizing the relative vector | the luciferase transgene was used for visualizing the relative vector | ||
distribution in all the animals in a real-time manner. This allows for in vivo imaging and time lapse experiments. | distribution in all the animals in a real-time manner. This allows for in vivo imaging and time lapse experiments. | ||
| - | === | + | ===Bioluminescence imaging=== |
| Line 74: | Line 74: | ||
==Methods== | ==Methods== | ||
| - | === | + | ===Contructs=== |
| - | === | + | ===Production of recombinant virus=== |
The viruses were produced in HEK 293-T cells and purified on an iodixanol gradient according to [https://2010.igem.org/Team:Heidelberg/Notebook/Methods#Virus_Production the virus production protocol]. | The viruses were produced in HEK 293-T cells and purified on an iodixanol gradient according to [https://2010.igem.org/Team:Heidelberg/Notebook/Methods#Virus_Production the virus production protocol]. | ||
Before infection, the titer of the viruses was quantified using [https://2010.igem.org/Team:Heidelberg/Notebook/Methods#Quantitative_Realtime_PCR quantitative realtime PCR]. | Before infection, the titer of the viruses was quantified using [https://2010.igem.org/Team:Heidelberg/Notebook/Methods#Quantitative_Realtime_PCR quantitative realtime PCR]. | ||
| - | === | + | ===Procedure involving animals=== |
The mouse experiments were conducted in accordance with the animal facility of the [https://2010.igem.org/Team:Heidelberg/Team/Institutes German Cancer Research Center in Heidelberg]. Female NMRI mice were obtained from a collaboration with Dr. Oliver Müller. At 8-10 weeks of age, the animals were injected in the tail vein (TV), with <nowiki>~</nowiki> 1x10<sup>11</sup> particles of AAV-SV40-luciferase in 200µl of 1x phosphate-buffered saline. The mice are transferred to a holding device which restrains the mouse while allowing access to the tail vein. The tails were warmed before the injections and injections were carried out using 27 gauge needles. All the mice recoverd from the injection quickly without loss of mobility or interruption of grooming activity {{HDref|Zincarelli et al., 2008}}. | The mouse experiments were conducted in accordance with the animal facility of the [https://2010.igem.org/Team:Heidelberg/Team/Institutes German Cancer Research Center in Heidelberg]. Female NMRI mice were obtained from a collaboration with Dr. Oliver Müller. At 8-10 weeks of age, the animals were injected in the tail vein (TV), with <nowiki>~</nowiki> 1x10<sup>11</sup> particles of AAV-SV40-luciferase in 200µl of 1x phosphate-buffered saline. The mice are transferred to a holding device which restrains the mouse while allowing access to the tail vein. The tails were warmed before the injections and injections were carried out using 27 gauge needles. All the mice recoverd from the injection quickly without loss of mobility or interruption of grooming activity {{HDref|Zincarelli et al., 2008}}. | ||
Revision as of 22:54, 27 October 2010

in vivo studyAbstractGene therapy offers a great tool for treatment of various diseases. Nevertheless it is only useful if it allows for specific, fine-tuning of the gene of interest (GOI). IntroductionGene therapy offers a great tool for treatment of various diseases such as failure, muscular dystrophies, and cystic fibrosis (Zincarelli, Soltys et al. 2008). ResultsConstructsThe in vivo analysis should enlighten our gene therapy approach using AAV tropism as well as miRNA binding sites as trigger for expression. The following constructs have been subcloned separately into the AAV context to accomplish those tasks:
Mice injectionThe tail vein injection was chosen so as to assess AAV serotype tissue tropism; the luciferase transgene was used for visualizing the relative vector distribution in all the animals in a real-time manner. This allows for in vivo imaging and time lapse experiments. Bioluminescence imagingDiscussionMethodsContructsProduction of recombinant virusThe viruses were produced in HEK 293-T cells and purified on an iodixanol gradient according to the virus production protocol. Before infection, the titer of the viruses was quantified using quantitative realtime PCR. Procedure involving animalsThe mouse experiments were conducted in accordance with the animal facility of the German Cancer Research Center in Heidelberg. Female NMRI mice were obtained from a collaboration with Dr. Oliver Müller. At 8-10 weeks of age, the animals were injected in the tail vein (TV), with ~ 1x1011 particles of AAV-SV40-luciferase in 200µl of 1x phosphate-buffered saline. The mice are transferred to a holding device which restrains the mouse while allowing access to the tail vein. The tails were warmed before the injections and injections were carried out using 27 gauge needles. All the mice recoverd from the injection quickly without loss of mobility or interruption of grooming activity [http://2010.igem.org/Team:Heidelberg/Project/Mouse_Infection#References (Zincarelli et al., 2008)]. in vivo animal imagingMice were anesthesized in an isofluran chamber. The mice were injected intraperitoneally with 200µl of a 30 mg/ml concentration of D-luciferin. This injection starts the luminescence of luc2. Mice were measured for one to seven minutes post injection under the in vivo bioluminometer. ReferencesGrimm, D. (2002). "Production methods for gene transfer vectors based on adeno-associated virus serotypes." Methods 28(2): 146-157.
|
|||
 "
"