Team:TU Delft/Safety/in-the-lab
From 2010.igem.org
(→Permit) |
(→Permit) |
||
| Line 16: | Line 16: | ||
Various degrees of containment are recognized, depending on the biosafety level needed for the genetically modified organism. The degree of containment is expressed as a level, ranging between ML1 to ML4, and each level required different safety precautions. Luckily, the organisms we worked with (''Escherichia coli'' and ''Pseudomonas putida'') were both level ML1 – the most harmless of GMOs. More information on how these levels are determined can be found in our Biosafety video in which we interviewed our biological safety officer. | Various degrees of containment are recognized, depending on the biosafety level needed for the genetically modified organism. The degree of containment is expressed as a level, ranging between ML1 to ML4, and each level required different safety precautions. Luckily, the organisms we worked with (''Escherichia coli'' and ''Pseudomonas putida'') were both level ML1 – the most harmless of GMOs. More information on how these levels are determined can be found in our Biosafety video in which we interviewed our biological safety officer. | ||
| - | At the Kuyver Laboratory for Biotechnology a permit to be allowed to work with GMOs is already present. To get permission for our experiments we had to supply a detailed research proposal to Dr. Robertson containing information on what strains and vectors we were going to use, which genetic modification we were going to make and which cultivation methods were going to be used. Only after approval of the research proposal could we start with our labwork - luckily she approved, and no changes had to be made. Because we are working under the general permit of the university we are also required to supply an end-report with what activities were performed and what the exact results were. | + | At the Kuyver Laboratory for Biotechnology a permit to be allowed to work with GMOs is already present. To get permission for our experiments we had to supply [https://static.igem.org/mediawiki/2010/c/c2/TU_Delft_research_proposal.pdf a detailed research proposal] to Dr. Robertson containing information on what strains and vectors we were going to use, which genetic modification we were going to make and which cultivation methods were going to be used. Only after approval of the research proposal could we start with our labwork - luckily she approved, and no changes had to be made. Because we are working under the general permit of the university we are also required to supply an end-report with what activities were performed and what the exact results were. |
[[Image:TUDelft_Handwashing.png|250px|left]] | [[Image:TUDelft_Handwashing.png|250px|left]] | ||
Revision as of 15:16, 27 October 2010
Biological Safety
Safety considerations are of great importance when working in a biological lab, and as an iGEM team working with biological material we took the necessary safety precautions very seriously.
Biosafety regulations in The Netherlands
In The Netherlands the Ministry of Housing, Spatial Planning and the Environment ([http://www.rijksoverheid.nl/international VROM]) is responsible for the regulations that protect the environment and human health during activities involving genetically modified organisms (GMOs) and is tasked with developing policy and regulations. Many of the implemented regulations concerning GMOs have been taken over from the EU legislation.
At the Kluyver laboratory for Biotechnology, according to what is stated by law, we have a biological safety officer, Dr. L.A. (Lesley) Robertson. The biological safety officer is in charge of day-to-day aspects of institutional biosafety and activities involving GMOs. She must monitor and ensure that all activities involving GMOs are executed in full conformity with Dutch legislation and regulations. An interview was done with Dr. Robertson which gives a clear insight into all the regulations at our laboratory – those regulated by law and those implemented by Dr. Robertson herself.
Permit
Prior to working with GMOs in the Netherlands you must obtain a ‘GMO permit’. The type of permit may vary, and the permit needed for iGEM was that of ‘Contained use’. This means that all activities involving GMOs are carried out in a confined area such as a laboratory and that there are no transboundary movements of the genetically modified materials.
Various degrees of containment are recognized, depending on the biosafety level needed for the genetically modified organism. The degree of containment is expressed as a level, ranging between ML1 to ML4, and each level required different safety precautions. Luckily, the organisms we worked with (Escherichia coli and Pseudomonas putida) were both level ML1 – the most harmless of GMOs. More information on how these levels are determined can be found in our Biosafety video in which we interviewed our biological safety officer.
At the Kuyver Laboratory for Biotechnology a permit to be allowed to work with GMOs is already present. To get permission for our experiments we had to supply a detailed research proposal to Dr. Robertson containing information on what strains and vectors we were going to use, which genetic modification we were going to make and which cultivation methods were going to be used. Only after approval of the research proposal could we start with our labwork - luckily she approved, and no changes had to be made. Because we are working under the general permit of the university we are also required to supply an end-report with what activities were performed and what the exact results were.
With knowledge comes responsibility
It is of great importance that everybody knows about the risks in a biological laboratory and can act accordingly and responsibly. This is why at our laboratory a number of tests have to be taken before you are allowed to work in the lab (unsupervised). The first of these is the Biosafety test: each individual is tested on their knowledge of the "Kluyver Biotechnology Survival Guide". This guide contains all information on personal safety, protective clothing, ML-1 regulations, what to do in case of a fire, the use of chemicals and chemical waste. By showing a number of photographs with 'wrong behavior' and asking the individual to identify what is wrong and why it is wrong an awareness is created amongst the new lab residence that will hopefully prevent them from making mistakes in the lab.
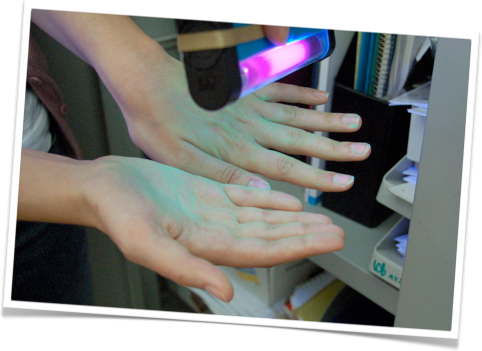
Another awareness-test is the hand-wash test devised by Dr. Robertson herself to make people aware of what they are carrying home on their hands after a day in lab (and not washing your hands).
We believe that such tests are very useful, and will create the right kind of mind-set required for each iGEM member to responsibly work in a biological lab. This is why we think it should be compulsory for each new iGEM member to take such a test and we are working on a quiz to perhaps use for future iGEM teams as well.
What is wrong with this picture?
The picture on the right is a representation of the other photo's also showed in the biosafety test. We dare you to find the 14 mistakes... Think you found all of them? Click on the image to see the mistakes.

Project-specific safety questions
From the start of our project we took in to consideration the possible safety issues our parts could raise. Because our work was limited to an ML-1 laboratory we were per definition not allowed to work with possibly dangerous or pathogenic genes and organisms, thus all parts we created are considered harmless.
When considering the safety of us, the researcher, while working on our iGEM project there were a few points that had to be taken into account. For the characterization of our BioBricks alkanes and alkanols were necessary. These materials are flammable, and therefore must be stored separately in a fireproof cabinet and must be handled with care in a flow cabinet. Furthermore we used E.coli strains, TOP 10 and K12, that are specifically weakened laboratory strains. These strains fall within the ML-1 classification, which indicated that they have a low (but not non-existent) virulence. Although organisms classified as Risk Group 1 are not known to consistently cause disease in healthy adults, young, elderly and immunocompromised individuals could have an increased risk of infection, but luckily all are team members can be considered healthy adults so there were not risks involved.
When thinking about public safety as well as environmental safety implications concerning our project, the choice of host strain was the most important factor. As just mentioned, our host strains, E.coli K12 and TOP10, are weakened organisms intended for laboratory use. Due to this, these strains could not compete with naturally occurring organisms outside of the laboratory and therefore do not pose a threat to the public or the environment. This is also the reason that our system can not be used for the intentioned goal (yet), the removal of hazardous/polluting oil in the environment, but hopefully more research will be done into this system in the future that will enable its safe use. For when this day has come, we have also considered the ethical implications about the implementation of our system for bio-remediation.
Biosafety in the Lab
In our Department at the TU Delft, the Department of Biotechnology, there is a Biological Safety Officer (BSO) who is responsible for the safety in the labs. In the Netherlands there is a very strict regulation about biosafety and working with genetically modified organisms. Our BSO, Lesley Robertson, always knows what biological material is being used, where and by whom. So, no better person to interview to get to know everything about biosafety!
 "
"