Team:Chiba/Circuit 1
From 2010.igem.org
| Line 228: | Line 228: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | <table border="0" cellpadding="30" cellspacing="0" | + | <table border="0" cellpadding="30" cellspacing="0" align="center"> |
<tr> | <tr> | ||
<td width="800px"><font size=2 face=verdana> | <td width="800px"><font size=2 face=verdana> | ||
Before injecting 2nd input, we must create none-input-environment. So we choose to wash the 1st input. By washing , tetR protein and cI434 protein will degrade. cI434 protein should disappear so that when there is 2nd input, T7 RNA Polymerase will be shown as a pulse which is the same as the 1st time. cI will begin to generate if tetR protein gets lost. We recognize it as time-limit, when there is enough cI generated (this means cI repression is stronger than T7 RNA Polymerase activation), there will be no GFP output. On the contrary, when there is less cI protein or no cI protein at the moment, T7 RNA Polymerase pulse can accumulate GFP output. By the second injected AHL before the inhibition by cI, T7 RNA Polymerase binds to the PT7/cI promoter and transcribes the downstream GFP. | Before injecting 2nd input, we must create none-input-environment. So we choose to wash the 1st input. By washing , tetR protein and cI434 protein will degrade. cI434 protein should disappear so that when there is 2nd input, T7 RNA Polymerase will be shown as a pulse which is the same as the 1st time. cI will begin to generate if tetR protein gets lost. We recognize it as time-limit, when there is enough cI generated (this means cI repression is stronger than T7 RNA Polymerase activation), there will be no GFP output. On the contrary, when there is less cI protein or no cI protein at the moment, T7 RNA Polymerase pulse can accumulate GFP output. By the second injected AHL before the inhibition by cI, T7 RNA Polymerase binds to the PT7/cI promoter and transcribes the downstream GFP. | ||
<center> | <center> | ||
| - | <img src="https://static.igem.org/mediawiki/2010/9/91/Chiba_planB_4.jpg" width=" | + | <img src="https://static.igem.org/mediawiki/2010/9/91/Chiba_planB_4.jpg" width="500px"><br> |
</center> | </center> | ||
</td> | </td> | ||
Revision as of 13:37, 27 October 2010

Overall Circuit  |
Fast Pulse |
| At initial state, LuxR and cI protein are constitutively generated. cI binds to the operator site of PT7/cI. When 1st input is injected, LuxR-AHL dimmer binds to the Lux-box of the lux promoters so that T7 RNA Polymerase, cI434 and tetR protein are generated at the same time. cI434 gradually accumulates, and gradually repress the transcription of T7 RNA Polymerase so that the expression of T7 RNA Polymerase can be shown as a pulse. At the same time, Transcription of cI is stopped by tetR, cI decomposes and the PT7/cI promoter is unbound. This derepression occurs after the pulse of T7 RNAP has passed. In other words, the operator sites of PT7/cI is repressed by cI when there is pulse of T7 RNA Polymerase. So, it cannot transcribe GFP. TetR creates a time delay here from input to derepression. Because of this time delay and one-time pulse, bacteria can never work by one input. |
Slow Pulse |
Before injecting 2nd input, we must create none-input-environment. So we choose to wash the 1st input. By washing , tetR protein and cI434 protein will degrade. cI434 protein should disappear so that when there is 2nd input, T7 RNA Polymerase will be shown as a pulse which is the same as the 1st time. cI will begin to generate if tetR protein gets lost. We recognize it as time-limit, when there is enough cI generated (this means cI repression is stronger than T7 RNA Polymerase activation), there will be no GFP output. On the contrary, when there is less cI protein or no cI protein at the moment, T7 RNA Polymerase pulse can accumulate GFP output. By the second injected AHL before the inhibition by cI, T7 RNA Polymerase binds to the PT7/cI promoter and transcribes the downstream GFP.
 |

This system consists of a pulse-generator and two inverters which can be seemed as a slow pulse.In this system, we also use AHL input and GFP output.
This time, we use AHL as an activate signal, so when there is AHL added, Lux promoter will be activated.
The transcription factors of output are T7 RNA Polymerase and cI repressor.
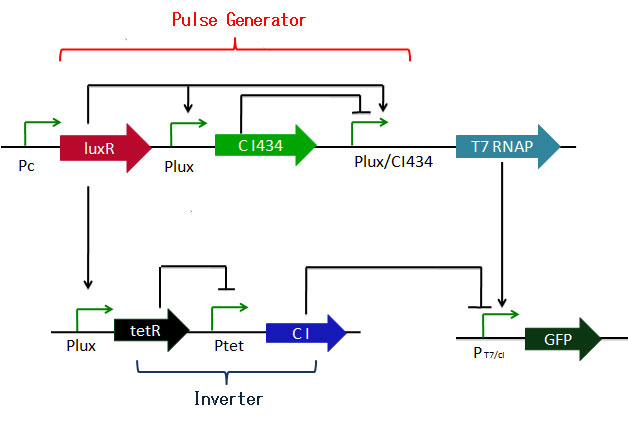
At initial state, LuxR and cI protein are constitutively generated.
cI binds to the operator site of PT7/cI.
When 1st input is injected, LuxR-AHL dimmer binds to the Lux-box of the lux promoters so that T7 RNA Polymerase, cI434 and tetR protein are generated
at the same time.
cI434 gradually accumulates, and gradually repress the transcription of T7 RNA Polymerase so that the expression of T7 RNA Polymerase can be shown
as a pulse.
At the same time, Transcription of cI is stopped by tetR, cI decomposes and the PT7/cI promoter is unbound.
This derepression occurs after the pulse of T7 RNAP has passed.
In other words, the operator sites of PT7/cI is repressed by cI when there is pulse of T7 RNA Polymerase.
So, it cannot transcribe GFP. TetR creates a time delay here from input to derepression.

Because of this time delay and one-time pulse, bacteria can never work by one input.

Before injecting 2nd input, we must create none-input-environment.
So we choose to wash the 1st input. By washing , tetR protein and cI434 protein will degrade.
cI434 protein should disappear so that when there is 2nd input, T7 RNA Polymerase will be shown as a pulse which is the same as the 1st time.
cI will begin to generate if tetR protein gets lost.
We recognize it as time-limit, when there is enough cI generated (this means cI repression is stronger than T7 RNA Polymerase activation),
there will be no GFP output.
On the contrary, when there is less cI protein or no cI protein at the moment, T7 RNA Polymerase pulse can accumulate GFP output.
By the second injected AHL before the inhibition by cI, T7 RNA Polymerase binds to the PT7/cI promoter and transcribes the downstream GFP.

 "
"