Team:Calgary/Project/CpxP
From 2010.igem.org
| Line 92: | Line 92: | ||
<p> | <p> | ||
The Cpx pathway is an outer membrane stress response system in <i>E. coli</i> that consists of two main components: CpxA, a sensor histidine kinase, and CpxR, a cytoplasmic response regulator (Raivio, Laird, Joly, & Silhavy, 2000). CpxA is a transmembrane protein that when induced, will phosphorylate CpxR, which is a transcriptional regulator of a large number of Cpx-induced proteins including chaperones and proteases associated with relieving the toxicity of misfolded protein (Keller & Hunke, 2009). This is achieved through the presence of a CpxR binding site (5’-GTAAAN<sub>5</sub>GTAAA-3’) present within 100 bp of the transcriptional start site of these genes (Price & Raivio, 2009). In the absence of induction signals, CpxP, a periplasmic protein, inhibits the autophosphorylating activity of CpxA, stopping the capability of CpxR to bind to these sites (Keller & Hunke, 2009). | The Cpx pathway is an outer membrane stress response system in <i>E. coli</i> that consists of two main components: CpxA, a sensor histidine kinase, and CpxR, a cytoplasmic response regulator (Raivio, Laird, Joly, & Silhavy, 2000). CpxA is a transmembrane protein that when induced, will phosphorylate CpxR, which is a transcriptional regulator of a large number of Cpx-induced proteins including chaperones and proteases associated with relieving the toxicity of misfolded protein (Keller & Hunke, 2009). This is achieved through the presence of a CpxR binding site (5’-GTAAAN<sub>5</sub>GTAAA-3’) present within 100 bp of the transcriptional start site of these genes (Price & Raivio, 2009). In the absence of induction signals, CpxP, a periplasmic protein, inhibits the autophosphorylating activity of CpxA, stopping the capability of CpxR to bind to these sites (Keller & Hunke, 2009). | ||
| - | + | <br/> | |
| - | + | ||
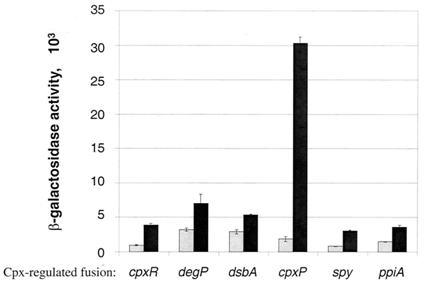
There are many stresses that are known to activate the Cpx pathway including elevated pH, overproduction of outer membrane lipoproteins such as NlpE, accumulation of pilus subunits, and the presence of misfolded proteins such as variants of maltose binding protein (Keller & Hunke, 2009). Though the exact response mechanism to each of the signals of the Cpx system is unknown, it has been proposed that each of these inducing signals act by causing the accumulation of misfolded proteins (Ruiz & Silhavy, 2005). Misfolded protein can titrate CpxP away from the periplasmic domain of CpxA, resulting in Cpx pathway activation (Raivio et al., 2000). Among the main proteins that are associated with outer membrane protein folding, CpxP shows the most sensitivity to Cpx activating conditions (Price & Raivio, 2009). It has also been shown that overexpression of CpxP will inhibit the activation of the Cpx pathway (MacRitchie et al., 2008). There are currently 50 genes that have shown experimental evidence for Cpx regulation (Price & Raivio, 2009). This year, we have constructed 3 Cpx inducible promoter regions to RFP reporter devices: CpxR, DegP, and CpxP. | There are many stresses that are known to activate the Cpx pathway including elevated pH, overproduction of outer membrane lipoproteins such as NlpE, accumulation of pilus subunits, and the presence of misfolded proteins such as variants of maltose binding protein (Keller & Hunke, 2009). Though the exact response mechanism to each of the signals of the Cpx system is unknown, it has been proposed that each of these inducing signals act by causing the accumulation of misfolded proteins (Ruiz & Silhavy, 2005). Misfolded protein can titrate CpxP away from the periplasmic domain of CpxA, resulting in Cpx pathway activation (Raivio et al., 2000). Among the main proteins that are associated with outer membrane protein folding, CpxP shows the most sensitivity to Cpx activating conditions (Price & Raivio, 2009). It has also been shown that overexpression of CpxP will inhibit the activation of the Cpx pathway (MacRitchie et al., 2008). There are currently 50 genes that have shown experimental evidence for Cpx regulation (Price & Raivio, 2009). This year, we have constructed 3 Cpx inducible promoter regions to RFP reporter devices: CpxR, DegP, and CpxP. | ||
| - | + | <br/> | |
| - | + | ||
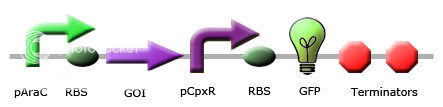
These circuits are designed with the purpose of detecting misfolding protein within the periplasm. If a gene of interest coding for a periplasmic protein is misfolded within the periplasm, it will activate the Cpx stress response pathway, and phosphorylated CpxR will be able to bind to our promoter regions, giving an RFP output. | These circuits are designed with the purpose of detecting misfolding protein within the periplasm. If a gene of interest coding for a periplasmic protein is misfolded within the periplasm, it will activate the Cpx stress response pathway, and phosphorylated CpxR will be able to bind to our promoter regions, giving an RFP output. | ||
Figure 2 shows characterization of a CpxR promoter region constructed upstream of an RFP reporter device put under stress from temperature. The results at 47°C show the best example of an induction of the Cpx pathway. An increase in temperature is a known Cpx induction signal, possibly resulting from the aggregation of misfolded protein in the periplasm (Ruiz & Silhavy, 2005). Therefore, CpxP leaving the periplasmic domain of CpxA, and phosphorylated CpxR acting on the CpxR promoter, which increases transcription of RFP, can explain the spike in RFP output within the first hour of stress. It is possible that the slower rate of increase of RFP between the first and third hours is a result of lowered phosphorylated CpxR in the periplasm, resulting from the accumulation of proteases, which are being overexpressed, in response to the toxicity of aggregation of misfolded protein. Because CpxP overproduction is known to inhibit the Cpx pathway activation, a large amount of it present within the periplasm could then show the decrease in RFP levels. The accumulation of CpxP, as well as other periplasmic proteases such as DegP could be alleviating stress caused by misfolded protein in the periplasm, allowing CpxP to remain bound to the transmembrane CpxA. | Figure 2 shows characterization of a CpxR promoter region constructed upstream of an RFP reporter device put under stress from temperature. The results at 47°C show the best example of an induction of the Cpx pathway. An increase in temperature is a known Cpx induction signal, possibly resulting from the aggregation of misfolded protein in the periplasm (Ruiz & Silhavy, 2005). Therefore, CpxP leaving the periplasmic domain of CpxA, and phosphorylated CpxR acting on the CpxR promoter, which increases transcription of RFP, can explain the spike in RFP output within the first hour of stress. It is possible that the slower rate of increase of RFP between the first and third hours is a result of lowered phosphorylated CpxR in the periplasm, resulting from the accumulation of proteases, which are being overexpressed, in response to the toxicity of aggregation of misfolded protein. Because CpxP overproduction is known to inhibit the Cpx pathway activation, a large amount of it present within the periplasm could then show the decrease in RFP levels. The accumulation of CpxP, as well as other periplasmic proteases such as DegP could be alleviating stress caused by misfolded protein in the periplasm, allowing CpxP to remain bound to the transmembrane CpxA. | ||
Revision as of 04:46, 27 October 2010

Cpx Circuits


The Cpx pathway is an outer membrane stress response system in E. coli that consists of two main components: CpxA, a sensor histidine kinase, and CpxR, a cytoplasmic response regulator (Raivio, Laird, Joly, & Silhavy, 2000). CpxA is a transmembrane protein that when induced, will phosphorylate CpxR, which is a transcriptional regulator of a large number of Cpx-induced proteins including chaperones and proteases associated with relieving the toxicity of misfolded protein (Keller & Hunke, 2009). This is achieved through the presence of a CpxR binding site (5’-GTAAAN5GTAAA-3’) present within 100 bp of the transcriptional start site of these genes (Price & Raivio, 2009). In the absence of induction signals, CpxP, a periplasmic protein, inhibits the autophosphorylating activity of CpxA, stopping the capability of CpxR to bind to these sites (Keller & Hunke, 2009).
There are many stresses that are known to activate the Cpx pathway including elevated pH, overproduction of outer membrane lipoproteins such as NlpE, accumulation of pilus subunits, and the presence of misfolded proteins such as variants of maltose binding protein (Keller & Hunke, 2009). Though the exact response mechanism to each of the signals of the Cpx system is unknown, it has been proposed that each of these inducing signals act by causing the accumulation of misfolded proteins (Ruiz & Silhavy, 2005). Misfolded protein can titrate CpxP away from the periplasmic domain of CpxA, resulting in Cpx pathway activation (Raivio et al., 2000). Among the main proteins that are associated with outer membrane protein folding, CpxP shows the most sensitivity to Cpx activating conditions (Price & Raivio, 2009). It has also been shown that overexpression of CpxP will inhibit the activation of the Cpx pathway (MacRitchie et al., 2008). There are currently 50 genes that have shown experimental evidence for Cpx regulation (Price & Raivio, 2009). This year, we have constructed 3 Cpx inducible promoter regions to RFP reporter devices: CpxR, DegP, and CpxP.
These circuits are designed with the purpose of detecting misfolding protein within the periplasm. If a gene of interest coding for a periplasmic protein is misfolded within the periplasm, it will activate the Cpx stress response pathway, and phosphorylated CpxR will be able to bind to our promoter regions, giving an RFP output.
Figure 2 shows characterization of a CpxR promoter region constructed upstream of an RFP reporter device put under stress from temperature. The results at 47°C show the best example of an induction of the Cpx pathway. An increase in temperature is a known Cpx induction signal, possibly resulting from the aggregation of misfolded protein in the periplasm (Ruiz & Silhavy, 2005). Therefore, CpxP leaving the periplasmic domain of CpxA, and phosphorylated CpxR acting on the CpxR promoter, which increases transcription of RFP, can explain the spike in RFP output within the first hour of stress. It is possible that the slower rate of increase of RFP between the first and third hours is a result of lowered phosphorylated CpxR in the periplasm, resulting from the accumulation of proteases, which are being overexpressed, in response to the toxicity of aggregation of misfolded protein. Because CpxP overproduction is known to inhibit the Cpx pathway activation, a large amount of it present within the periplasm could then show the decrease in RFP levels. The accumulation of CpxP, as well as other periplasmic proteases such as DegP could be alleviating stress caused by misfolded protein in the periplasm, allowing CpxP to remain bound to the transmembrane CpxA.
 "
"