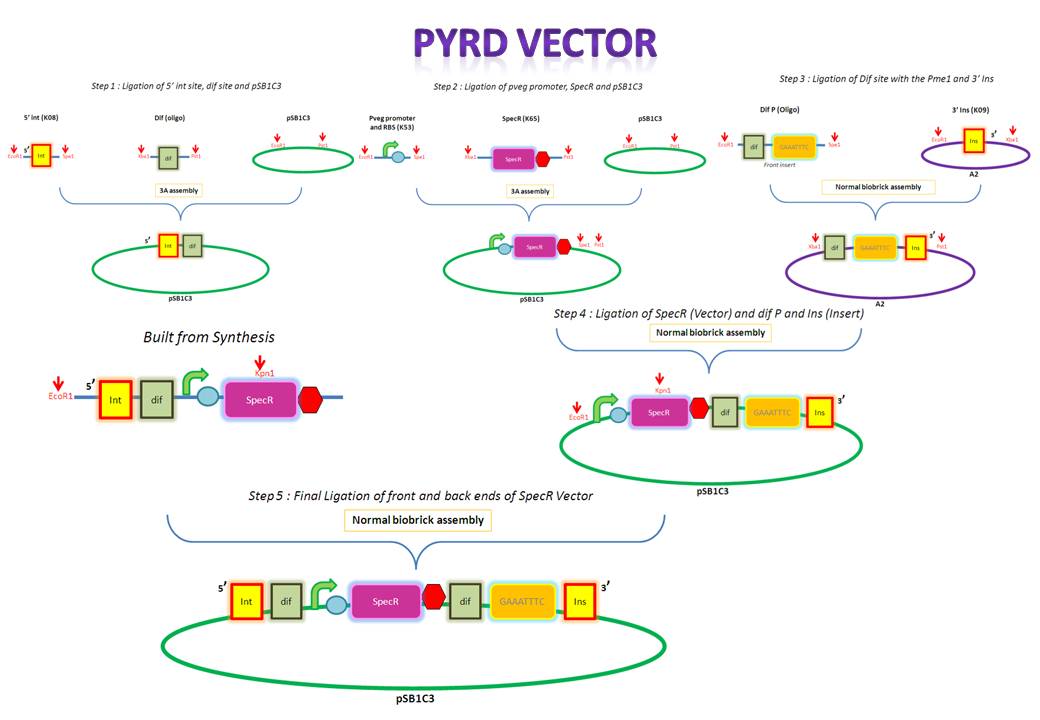
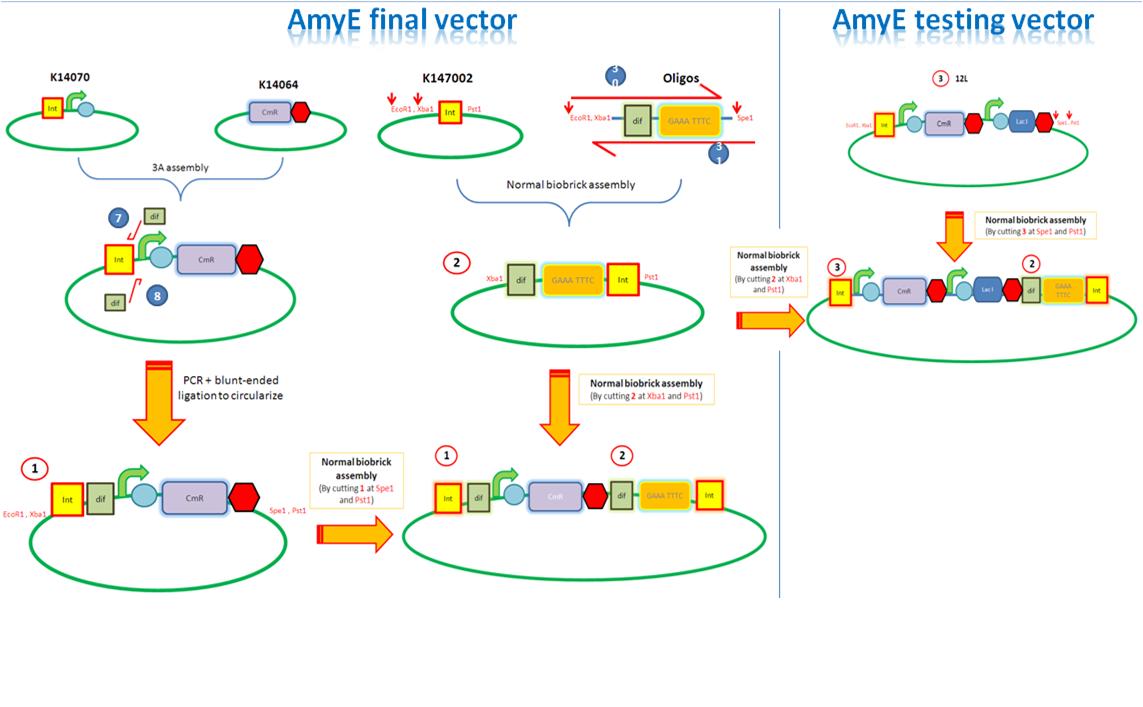
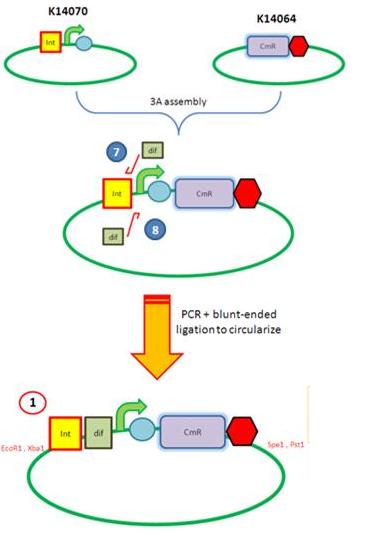
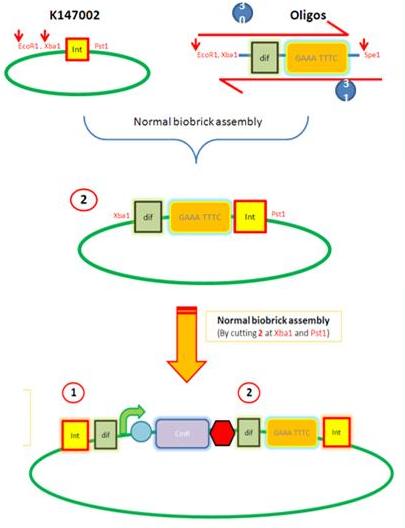
|
|
| Line 1,077: |
Line 1,077: |
| | </html> | | </html> |
| | | | |
| - | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1"
| |
| - |
| |
| - | |-
| |
| - | ! Week 7 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday
| |
| - | |-
| |
| - | | MORNING
| |
| - | || '''Starting assembly of AmyE vector'''
| |
| - | * Restriction digestion of BB k14070 for the AmyE vector (using Eco and Spe)
| |
| - | * Restriction digestion of BB k14064 for the AmyE vector (using Eco and Xba)
| |
| - | * Restriction digestion of 5' integration site (k08) for the PyrD vector (using Eco and Spe)
| |
| - | * PCR amplification of vector backbone PSB1C3 for the PyrD vector
| |
| - | ||
| |
| - | * Gel analysis and extraction of 5' int site for PyrD vector (repetition of step due to absence of DNA during gel analysis yesterday)
| |
| - | * Gel purification of k70
| |
| - | * Gel purification of k08
| |
| - | * Re-analysis of k70, k64 (for AmyE) on gel to work out ratios for ligation set up
| |
| - | * Re-analysis of k08, Pme oligos and pSB1C3 (for PyrD) on gel to work out ratios for ligation set up
| |
| - | * Restriction digestion of pveg promoter (k53) (using Eco and Spe) and spec cassette (k65) (using Xba and Pst) for PyrD vector
| |
| - | * Restriction digestion of pSB1C3 for PyrD (using Eco and Pst)
| |
| - | ||
| |
| - | * Check for transformed colonies (colonies that have taken up the vector with the 5'diff) and prepare for colony PCR
| |
| - | * Gel purification of k53 and k65 for AmyE in preparation for ligations this afternoon
| |
| - | * Gel extraction and re-analysis on gel of k53, k65 and psB1C3 for Spec casette in preparation for ligations this afternoon
| |
| - | ||
| |
| - | * Transformation of overnight ligations of:
| |
| - | k64 and k70, k70 only,
| |
| - |
| |
| - | Spec
| |
| - |
| |
| - | and 5' PyrD diff
| |
| - | ||
| |
| - | * Replica plating and colony PCR of:
| |
| - | Spec and 5' PyrD diff
| |
| - | * Plate wash of:
| |
| - | k64 and k70. k70 only was discarded since this was purely for a baground check
| |
| - | |-
| |
| - | | AFTERNOON
| |
| - | ||
| |
| - | * PCR purification of PSB1C3 vector
| |
| - | * PCR purification of BB k14064 digestion products
| |
| - | * Gel analysis of digestion products of BB k14070
| |
| - | * Gel extraction of digestion products of BB k14070
| |
| - | ||
| |
| - | * Restriction digestion of k64 and subsequent PCR purification (repetition of step due to absence of DNA during gel analysis)
| |
| - | * Gel analysis of k70, k64 (for AmyE) to work out ligation ratios
| |
| - | * Gel analysis and extraction of k53 and k65
| |
| - | * Bench (1 hour) and overnight ligation of 5'diff with the pSB1C3 vector
| |
| - | * Transformation of E.Coli with the bench ligated vector
| |
| - | ||
| |
| - | * Dephosphorylation of k64 and set up of overnight ligations for k64 and k70 (vector and insert) and k64 (vector)only (for negative control; check of background)
| |
| - | * Set up overnight ligations of SpecR
| |
| - | ||
| |
| - | ||
| |
| - | * Gel analysis of colony PCR products of Spec and 5' PyrD diff
| |
| - | * Annealing of diff P oligos (used for both PyrD and AmyE vectors)
| |
| - | |}
| |
| | |} | | |} |
| | | | |
| Line 1,257: |
Line 1,201: |
| | </html> | | </html> |
| | | | |
| - | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1"
| |
| - |
| |
| - | |-
| |
| - | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday !! Saturday
| |
| - | |-
| |
| - | | MORNING
| |
| - | ||
| |
| - | Set up overnight ligations for standard assembly (BBA) and 3A cloning (3A) of dif P
| |
| - | * Restriction digestion of 3' integration site (K02) in the A2 vector ( Using Eco and Xba) for BBA
| |
| - | * Restriction digestion of 3' integration site (K09) in the AK3 vector ( Using Eco and Xba) for BBA
| |
| - | * PCR purification of 3' integration site (K02) in the A2 vector ( Using Eco and Xba) for BBA
| |
| - | * PCR purification of 3' integration site (K09) in the AK3 vector ( Using Eco and Xba) for BBA
| |
| - | * Set up 5 ml culture of spec from colony 1 of replica plate in shaking incubator @ 37 degrees
| |
| - |
| |
| - | ||
| |
| - | * Restriction digestion of 3' integration site (K02) in the A2 vector ( Using Xba and Pst) for 3A
| |
| - | * Restriction digestion of 3' integration site (K09) in the AK3 vector ( Using Xba and Pst) for 3A
| |
| - | ||
| |
| - | * Transformations using the overnight ligations showed a lot of background. Therefore we set up ligations for K02 and K09 using 3A cloning. The results will show if this method is preferable due to less background.
| |
| - | * Midiprep of CmR vector and test digest using Eco and Spe
| |
| - |
| |
| - | * Backbone PCR of PSB1C3 vector (1st attempt) for common use
| |
| - |
| |
| - |
| |
| - | ||
| |
| - | * Replica plating of transformed colonies for k09 from the plate with the insert (diff P) - 45 sigle colonies were plated
| |
| - | * The first 15 of the above colonies were colony PCRed using dif PES Fwd and pSB Rev
| |
| - | ||
| |
| - | * Gel analysis of colony PCR from yesterday (first 15 replica plated colonies
| |
| - | * Set up further colony PCR reactions for the same 15 colonies using pSB Fwd and pSB Rev primers
| |
| - | * Gel analysis of pSB1C3 - after backbone PCR and after digestion (looked contaminated!)
| |
| - | * Test digests of K54 K70 Midi prep using i) EcoRI, ii) SpeI and iii) EcoRI + SpeI
| |
| - | * Screen next 20 colonies by colony PCR, use higher temperatures to avoid previous non specific annealing
| |
| - | ||
| |
| - | * Miniprep of 4 overnight cultures (dif P and 3' Insert for PyrD) - colonies 4,5,7 & 9
| |
| - | * Run Colony PCR results on a Gel - pick promissing candidates for mini prep.
| |
| - |
| |
| - | |-
| |
| - | | AFTERNOON
| |
| - | ||
| |
| - | * Gel analysis of PCR purified K02 and K09 with the insert (diff P) in between to work out ratios for the liagation
| |
| - | * Dephosphorylation of digested A2 vector with 3' integration site (K02)
| |
| - | * Dephosphorylation of digested AK3 vector with 3' integration site (K09)
| |
| - | * Set up overnight ligation of A2 vector with 3' integration site (K02) with diff P (insert) and A2 vector only
| |
| - | * Set up overnight ligation of AK3 vector with 3' integration site (K09) with diff P (insert) and AK3 vector only
| |
| - | * Colony PCR and gel analysis of plated culture (from plate wash on Friday) with K64 and K70
| |
| - | * Overnight 100 ml culture of spec @ 14 degrees
| |
| - | ||
| |
| - | * Electroporation of the 4 overnight ligations described on Thursday afternoon
| |
| - | * Gel extraction of the digestion products ( 3' integration sites - now our inserts) described this morning for 3A
| |
| - | * Gel analysis of gel extracted K02 nad K09 (inserts) with diff P (also an insert) and pSB1C3 (vector) in between
| |
| - | * Set up overnight 100 ml culture of CmR vector (K64 and K70) for midiprep tomorrow
| |
| - | * Set up overnight culture plates (AmpR) for the 4 electroporated cultures (colonies that survive will contain transformed cells
| |
| - |
| |
| - | ||
| |
| - | * PCR purification of PSB1C3 PCR amplified vector
| |
| - | * Check concentration of midi prepped CmR
| |
| - | * Run a gel to visualise the results - Gel contained pSB1C3 (PCR purified) , CmR (Midiprepped) and CmR digested (Midiprepped)
| |
| - |
| |
| - | ||
| |
| - | * Backbone PCR of pSB1C3 (2nd attempt), PCR purified and then digested with Eco and Pst
| |
| - | * Midipreps sent for sequencing ( Spec and CmR)
| |
| - | ||
| |
| - | Backbone PCR of pSB1C3 using PFU (3rd attempt)
| |
| - | * Set up overnight 5 ml cultures for miniprepping tomorrow - 4 cultures were set up by looking at the gel this morning; 2 positive looking (4 & 7), 1 negative (5) and one containing nothing (9)
| |
| - | ||
| |
| - | * Diagnostic digests of minipreps - Two digests : One with Spe & Pst and other with Xba & Spe
| |
| - | |}
| |
| | |} | | |} |
| | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" |
| Line 1,421: |
Line 1,297: |
| | </html> | | </html> |
| | | | |
| - | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1"
| |
| | | | |
| - | |-
| |
| - | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday
| |
| - | |-
| |
| - | | MORNING
| |
| - | ||
| |
| - | * Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | * Test Digest of Mini Prep K02+dif EcoRI and SpeI
| |
| - |
| |
| - | ||
| |
| - | * Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | * Restriction digest of K02 from registrty for 3A assembly
| |
| - | ||
| |
| - | * Gel purification of insert (35 ul)
| |
| - | * Gel analysis of vector and insert to work out ratios for ligations
| |
| - | ||
| |
| - | * Transformation with overnight ligations
| |
| - | * PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | * Gel Purified K02 and PBB1C3 digests
| |
| - | ||
| |
| - | * The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies
| |
| - | * 10 individual colonies were replica plated and used for colony PCR
| |
| - | * Dephosphorylate PSB1C3 using alkaline phosphotase
| |
| - | ||
| |
| - | |-
| |
| - | | AFTERNOON
| |
| - | ||
| |
| - | * Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
| |
| - | * Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.
| |
| - | ||
| |
| - | * Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
| |
| - | * PCR purification of vetor (35 ul)
| |
| - | * Gel extraction of insert after gel analysis (35 ul)
| |
| - | * Midi prep of sample 8 K54 + K70
| |
| - | ||
| |
| - | * Dephosphorylation of vector
| |
| - | * Set up two overnight ligations; Vector & Insert and Vector only
| |
| - | ||
| |
| - | * Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight
| |
| - | ||
| |
| - | * Gel analysis of colony PCR products
| |
| - | * Set up ligation reaction K02+dif using 3A method for Transformation on Monday.
| |
| - | |}
| |
| | |} | | |} |
| | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" |
| Line 1,470: |
Line 1,303: |
| | |- | | |- |
| | | | | | |
| - | <html>
| |
| - | <table width="850px" border="0">
| |
| - | <tr>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <b>Day</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Monday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Tuesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Wednesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Thursday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Friday</b>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;width:100px;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Morning</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | <li>Test Digest of Mini Prep K02+dif EcoRI and SpeI</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | <li>Restriction digest of K02 from registrty for 3A assembly.</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel purification of insert (35 ul)
| |
| - | <li>Gel analysis of vector and insert to work out ratios for ligations </li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Transformation with overnight ligations
| |
| - | <li>PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | <li>Gel Purified K02 and PBB1C3 digests</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies
| |
| - | <li>10 individual colonies were replica plated and used for colony PCR
| |
| - | <li>Dephosphorylate PSB1C3 using alkaline phosphotase</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Afternoon</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
| |
| - | <li>Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
| |
| - | <li>PCR purification of vetor (35 ul)
| |
| - | <li>Gel extraction of insert after gel analysis (35 ul)
| |
| - | <li>Midi prep of sample 8 K54 + K70</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Dephosphorylation of vector
| |
| - | <li>Set up two overnight ligations; Vector & Insert and Vector only</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Both ligations ('Insert & Vector' and '''Vector only''') were plated in CmR and incubated overnight</li>
| |
| | | | |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel analysis of colony PCR products
| |
| - | <li>Set up ligation reaction K02+dif using 3A method for Transformation on Monday.</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | </table>
| |
| - | </html>
| |
| - |
| |
| - | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1"
| |
| - |
| |
| - | |-
| |
| - | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday
| |
| - | |-
| |
| - | | MORNING
| |
| - | ||
| |
| - | * Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | * Test Digest of Mini Prep K02+dif EcoRI and SpeI
| |
| - |
| |
| - | ||
| |
| - | * Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | * Restriction digest of K02 from registrty for 3A assembly.
| |
| - | ||
| |
| - | * Gel purification of insert (35 ul)
| |
| - | * Gel analysis of vector and insert to work out ratios for ligations
| |
| - | ||
| |
| - | * Transformation with overnight ligations
| |
| - | * PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | * Gel Purified K02 and PBB1C3 digests
| |
| - | ||
| |
| - | * The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies
| |
| - | * 10 individual colonies were replica plated and used for colony PCR
| |
| - | * Dephosphorylate PSB1C3 using alkaline phosphotase
| |
| - | ||
| |
| - | |-
| |
| - | | AFTERNOON
| |
| - | ||
| |
| - | * Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
| |
| - | * Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.
| |
| - | ||
| |
| - | * Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
| |
| - | * PCR purification of vetor (35 ul)
| |
| - | * Gel extraction of insert after gel analysis (35 ul)
| |
| - | *Midi prep of sample 8 K54 + K70
| |
| - | ||
| |
| - | * Dephosphorylation of vector
| |
| - | * Set up two overnight ligations; Vector & Insert and Vector only
| |
| - | ||
| |
| - | * Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight
| |
| - | ||
| |
| - | * Gel analysis of colony PCR products
| |
| - | * Set up ligation reaction K02+dif using 3A method for Transformation on Monday.
| |
| - | |}
| |
| | | | |
| | |} | | |} |
| Line 1,612: |
Line 1,310: |
| | |- | | |- |
| | | | | | |
| - | <html>
| |
| - | <table width="850px" border="0">
| |
| - | <tr>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <b>Day</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Monday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Tuesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Wednesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Thursday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Friday</b>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;width:100px;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Morning</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Afternoon</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | <li></li>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | </table>
| |
| - | </html>
| |
| | | | |
| - | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1"
| |
| - |
| |
| - | |-
| |
| - | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday
| |
| - | |-
| |
| - | | MORNING
| |
| - | ||
| |
| - | * Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | * Test Digest of Mini Prep K02+dif EcoRI and SpeI
| |
| - |
| |
| - | ||
| |
| - | * Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | * Restriction digest of K02 from registrty for 3A assembly.
| |
| - | ||
| |
| - | * Gel purification of insert (35 ul)
| |
| - | * Gel analysis of vector and insert to work out ratios for ligations
| |
| - | ||
| |
| - | * Transformation with overnight ligations
| |
| - | * PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | * Gel Purified K02 and PBB1C3 digests
| |
| - | ||
| |
| - | * The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies
| |
| - | * 10 individual colonies were replica plated and used for colony PCR
| |
| - | * Dephosphorylate PSB1C3 using alkaline phosphotase
| |
| - | ||
| |
| - | |-
| |
| - | | AFTERNOON
| |
| - | ||
| |
| - | * Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
| |
| - | * Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.
| |
| - | ||
| |
| - | * Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
| |
| - | * PCR purification of vetor (35 ul)
| |
| - | * Gel extraction of insert after gel analysis (35 ul)
| |
| - | * Midi prep of sample 8 K54 + K70
| |
| - | ||
| |
| - | * Dephosphorylation of vector
| |
| - | * Set up two overnight ligations; Vector & Insert and Vector only
| |
| - | ||
| |
| - | * Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight
| |
| - | ||
| |
| - | * Gel analysis of colony PCR products
| |
| - | * Set up ligation reaction K02+dif using 3A method for Transformation on Monday.
| |
| - | |}
| |
| | | | |
| | |} | | |} |
| Line 1,743: |
Line 1,317: |
| | |- | | |- |
| | | | | | |
| - | <html>
| |
| - | <table width="850px" border="0">
| |
| - | <tr>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <b>Day</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Monday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Tuesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Wednesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Thursday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Friday</b>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;width:100px;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Morning</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | <li>Test Digest of Mini Prep K02+dif EcoRI and SpeI</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | <li>Restriction digest of K02 from registrty for 3A assembly.</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel purification of insert (35 ul)
| |
| - | <li>Gel analysis of vector and insert to work out ratios for ligations</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Transformation with overnight ligations</font>
| |
| - | <li>PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | <li>Gel Purified K02 and PBB1C3 digests</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Afternoon</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | <li></li>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li></li>
| |
| - | <li></li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | </table>
| |
| - | </html>
| |
| | | | |
| - | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1"
| |
| - |
| |
| - | |-
| |
| - | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday
| |
| - | |-
| |
| - | | MORNING
| |
| - | ||
| |
| - | * Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | *Test Digest of Mini Prep K02+dif EcoRI and SpeI
| |
| - |
| |
| - | ||
| |
| - | * Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | * Restriction digest of K02 from registrty for 3A assembly.
| |
| - | ||
| |
| - | * Gel purification of insert (35 ul)
| |
| - | * Gel analysis of vector and insert to work out ratios for ligations
| |
| - | ||
| |
| - | * Transformation with overnight ligations</font>
| |
| - | *PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | *Gel Purified K02 and PBB1C3 digests
| |
| - | ||
| |
| - | * The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies
| |
| - | * 10 individual colonies were replica plated and used for colony PCR
| |
| - | * Dephosphorylate PSB1C3 using alkaline phosphotase
| |
| - | ||
| |
| - | |-
| |
| - | | AFTERNOON
| |
| - | ||
| |
| - | * Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
| |
| - | * Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.
| |
| - | ||
| |
| - | * Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
| |
| - | * PCR purification of vetor (35 ul)
| |
| - | * Gel extraction of insert after gel analysis (35 ul)
| |
| - | * Midi prep of sample 8 K54 + K70
| |
| - | ||
| |
| - | * Dephosphorylation of vector
| |
| - | * Set up two overnight ligations; Vector & Insert and Vector only
| |
| - | ||
| |
| - | * Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight
| |
| - | ||
| |
| - | * Gel analysis of colony PCR products
| |
| - | * Set up ligation reaction K02+dif using 3A method for Transformation on Monday.
| |
| - | |}
| |
| | | | |
| | |} | | |} |
| Line 1,960: |
Line 1,405: |
| | </html> | | </html> |
| | | | |
| - | <html>
| |
| - | <table width="850px" border="0">
| |
| - | <tr>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <b>Day</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Monday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Tuesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Wednesday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Thursday</b>
| |
| - | </td>
| |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Friday</b>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;width:100px;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Morning</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | <li>Test Digest of Mini Prep K02+dif EcoRI and SpeI</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | <li>Restriction digest of K02 from registrty for 3A assembly.</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel purification of insert (35 ul)
| |
| - | <li>Gel analysis of vector and insert to work out ratios for ligations</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Transformation with overnight ligations
| |
| - | <li>PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | <li>Gel Purified K02 and PBB1C3 digests</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>The transformations were highly successful!! The 'Vector only' plate showed no colonies and the 'Insert & Vector' plate showed many individual colonies
| |
| - | <li>10 individual colonies were replica plated and used for colony PCR
| |
| - | <li>Dephosphorylate PSB1C3 using alkaline phosphotase</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | <tr>
| |
| - | <td style="background-color:#FFCC66;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Afternoon</b>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
| |
| - | <li>Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
| |
| - | <li>PCR purification of vetor (35 ul)
| |
| - | <li>Gel extraction of insert after gel analysis (35 ul)
| |
| - | <li>Midi prep of sample 8 K54 + K70</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Dephosphorylation of vector
| |
| - | <li>Set up two overnight ligations; Vector & Insert and Vector only</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| |
| - | <ul>
| |
| - | <li>Gel analysis of colony PCR products
| |
| - | <li>Set up ligation reaction K02+dif using 3A method for Transformation on Monday.</li>
| |
| - | </ul>
| |
| - | </td>
| |
| - | </tr>
| |
| - | </table>
| |
| - | </html>
| |
| - | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1"
| |
| | | | |
| - | |-
| |
| - | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday
| |
| - | |-
| |
| - | | MORNING
| |
| - | ||
| |
| - | * Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
| |
| - | * Test Digest of Mini Prep K02+dif EcoRI and SpeI
| |
| - |
| |
| - | ||
| |
| - | * Midiprep of Colony 4 - concentration 110 ng/ul
| |
| - | * Restriction digest of K02 from registrty for 3A assembly.
| |
| - | ||
| |
| - | * Gel purification of insert (35 ul)
| |
| - | * Gel analysis of vector and insert to work out ratios for ligations
| |
| - | ||
| |
| - | * Transformation with overnight ligations
| |
| - | * PCR amplified the PSB1C3 vector for 3A assembly
| |
| - | * Gel Purified K02 and PBB1C3 digests
| |
| - | ||
| |
| - | * The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies
| |
| - | * 10 individual colonies were replica plated and used for colony PCR
| |
| - | * Dephosphorylate PSB1C3 using alkaline phosphotase
| |
| - | ||
| |
| - | |-
| |
| - | | AFTERNOON
| |
| - | ||
| |
| - | * Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
| |
| - | * Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.
| |
| - | ||
| |
| - | * Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
| |
| - | * PCR purification of vetor (35 ul)
| |
| - | * Gel extraction of insert after gel analysis (35 ul)
| |
| - | *Midi prep of sample 8 K54 + K70
| |
| - | ||
| |
| - | * Dephosphorylation of vector
| |
| - | * Set up two overnight ligations; Vector & Insert and Vector only
| |
| - | ||
| |
| - | * Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight
| |
| - | ||
| |
| - | * Gel analysis of colony PCR products
| |
| - | * Set up ligation reaction K02+dif using 3A method for Transformation on Monday.
| |
| - | |}
| |
| | |} | | |} |
 "
"