Team:Harvard/flavor/notebook
From 2010.igem.org
(→06-16-2010 [ top ]) |
(→06-17-2010 [ top ]) |
||
| Line 105: | Line 105: | ||
==<html><a class="labnotebook" name="06-17-2010">06-17-2010</a></html> [ [[#top|top]] ]== | ==<html><a class="labnotebook" name="06-17-2010">06-17-2010</a></html> [ [[#top|top]] ]== | ||
| + | ===PCR Purification of pORE Vector Parts=== | ||
| + | Following QIAgen PCR Purification Kit Protocol the following PCR products were purified: | ||
| + | #pENTCUP2 Promoter - 16.1 ng/μL | ||
| + | #NOSterminator Sequence - 76.3 ng/μL | ||
| + | #STOP codon + NOSterminator Sequence - 76.5 ng/μL | ||
| + | To do: follow up with restriction digest <font color="green"> ✓ </font> and Agarose gel<font color="green"> ✓ </font> to confirm PCR and PCR purification. <!--<font color="green"> ✓ </font>--> | ||
| + | |||
| + | ===Restriction Digest and Agarose Gel=== | ||
| + | Restriction Digest reactions were set up as follows: | ||
| + | |||
| + | pENTCUP2 Promoter:<br> | ||
| + | *27μL PCR product<br> | ||
| + | *3μL Loading Buffer w/ dye<br> | ||
| + | *1μL Xba1 Fast Enzyme<br> | ||
| + | *1μL Pst1 Fast Enzyme<br> | ||
| + | |||
| + | NOSterm and NOSterm + STOP: | ||
| + | *9μL PCR product | ||
| + | *1μL Loading Buffer w/ dye | ||
| + | *0.5μL Xba1 Fast Enzyme | ||
| + | *0.5μL Pst1 Fast Enzyme | ||
| + | |||
| + | BioBrick Plasmid V0120 (Ampicillin Resistance): | ||
| + | *3μL DNA | ||
| + | *1μL Loading Buffer w/ dye | ||
| + | *6μL diH<sub>2</sub>O | ||
| + | *0.5μL Xba1 Fast Enzyme | ||
| + | *0.5μL Pst1 Fast Enzyme | ||
| + | |||
| + | Reactions were allowed to proceed for 45 minutes at 37°C.<br> | ||
==<html><a class="labnotebook" name="06-18-2010">06-18-2010</a></html> [ [[#top|top]] ]== | ==<html><a class="labnotebook" name="06-18-2010">06-18-2010</a></html> [ [[#top|top]] ]== | ||
Revision as of 00:28, 25 October 2010

notebook calendar [ top ]
06-14-2010 [ top ]
- Miraculin and brazzein constructs due to arrive on Wednesday from Mr. Gene
BioBrick Transformation
BioBrick parts from the 2010 iGEM kit were transformed and grown in highly competent TURBO bacteria.
- Wintergreen Scent Pathway:
BBa_J45700 - entire pathway, Ampicillin
BBa_J45004 - BSMT1 only, Ampicillin
(Not in 2010 BB Kit: BBa_J45017 - PchB, PchA)
- Banana Scent Pathway:
BBa_J45250 - ATF3 + Promoter, Ampicillin?
BBa_J45014 - ATF3 only, Ampicillin
(Not in BB 2010 Kit: BBa_J45400 - BAT2 and THI3)
06-15-2010 [ top ]
Primer Designs for Agrobacterium Vector
- Primers were designed to amplify from the Expression Series pORE Agrobacterium plasmid (e3) 1)the pENTcup2 promoter, 2) the tNOS stop sequence, 3) the tNOS stop sequence + additional stop codon and 4) the pHLP promoter.
Note: the NOSterm_BB_R primer works for both tNOS and tNOS+STOP codon sequences.
pENTcup2_BB_F CCTTTCTAGAGGGATCTTCTGCAAGCATCT
pENTcup2_BB_R AAGGCTGCAGCGGCCGCTACTAGTTCCGGTGGGTTTTGAGGT
STOP_NOSterm_BB_F CCTTTCTAGATGAGATCGTTCAAACATTTGG
NOSterm_BB_F CCTTTCTAGAGATCGTTCAAACATTTGGCA
NOSterm_BB_R AAGGCTGCAGCGGCCGCTACTAGTGATCTAGTAACATAGATGACA
pHPL_BB_F CCTTTCTAGAAACGTGGATACTTGGCAGTG
pHPL_BB_R AAGGCTGCAGCGGCCGCTACTAGTCTTTTGAGCTTAGAGGTTTTT
BioBrick Transformation
- Colonies were observed after overnight culture growth, but in low quantities.
- J45700 and J45004 (both Wintergreen Pathway) showed minimal number of colonies on both 10μL and 100μL cultures.
- J45250 and J45014 (both Banana Pathway) showed no colonies on either the 10μL or 100μL cultures.
Codon Usage in Arabidopsis
Using http://gcua.schoedl.de/
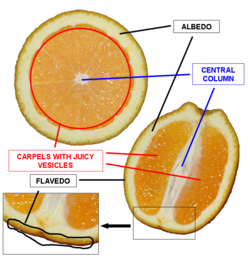
Valencene Extraction
Used RNeasy Plant Mini Kit to extract RNA from the flavedo of an Organic Valencia Orange.
06-16-2010 [ top ]
MiniPrep
- MiniPrep of Wintergreen parts from Registry (J45004 and J45700) following Qiagen MiniPrep protocol. MiniPrep three samples of each part.
- DNA concentrations:
J45004-1: 59.1 ng/μL
J45004-2: 72.9 ng/μL
J45004-3: 88.1 ng/μL
J45700-1: 146.7 ng/μL
J45700-2: 187.4 ng/μL
J45700-3: 175.5 ng/μL
Digestion with Enzymes
- For J45004, we added: 9μL DNA, 1μL buffer, 1μL xbaI restriction enzyme (slow), and .5μL pstI restriction enzyme (fast).
- For J45700, we added 5μL DNA, 4μL H2O, 1μL buffer, 1μL xbaI restriction enzyme (slow), and .5μL pstI restriction enzyme (fast).
- We let mixtures sit for 30 minutes due to the use of xbaI restriction enzyme (slow). After the 30 minutes, we added 2.5μL dye to each mix.
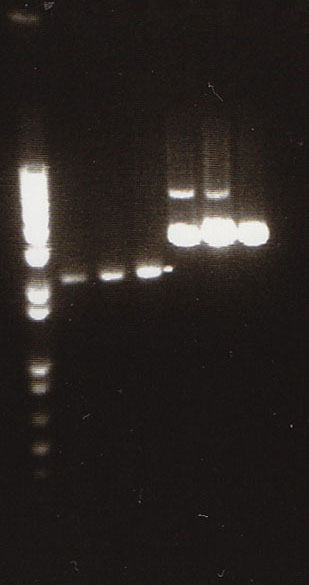
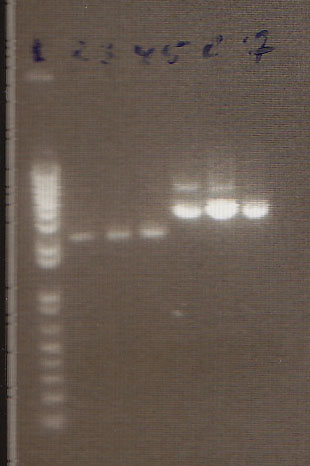
Gel
- We loaded 12.5μL of 1kb ladder to well 1 of the gel (numbered left to right). We then loaded 12.5μL of J45004-1, J45004-2, and J45004-3 to wells 2,3, and 4, respectively. We loaded J45700-1, J45700-2, J4500-3 in wells 5, 6, and 7, respectively (see images below for well locations).
- Ran on 1% agarose gel.
06-17-2010 [ top ]
PCR Purification of pORE Vector Parts
Following QIAgen PCR Purification Kit Protocol the following PCR products were purified:
- pENTCUP2 Promoter - 16.1 ng/μL
- NOSterminator Sequence - 76.3 ng/μL
- STOP codon + NOSterminator Sequence - 76.5 ng/μL
To do: follow up with restriction digest ✓ and Agarose gel ✓ to confirm PCR and PCR purification.
Restriction Digest and Agarose Gel
Restriction Digest reactions were set up as follows:
pENTCUP2 Promoter:
- 27μL PCR product
- 3μL Loading Buffer w/ dye
- 1μL Xba1 Fast Enzyme
- 1μL Pst1 Fast Enzyme
NOSterm and NOSterm + STOP:
- 9μL PCR product
- 1μL Loading Buffer w/ dye
- 0.5μL Xba1 Fast Enzyme
- 0.5μL Pst1 Fast Enzyme
BioBrick Plasmid V0120 (Ampicillin Resistance):
- 3μL DNA
- 1μL Loading Buffer w/ dye
- 6μL diH2O
- 0.5μL Xba1 Fast Enzyme
- 0.5μL Pst1 Fast Enzyme
Reactions were allowed to proceed for 45 minutes at 37°C.
 "
"