Team:Groningen/23 August 2010
From 2010.igem.org
| Line 2: | Line 2: | ||
<br> | <br> | ||
| - | + | ||
<br> | <br> | ||
| - | + | ||
| + | '''Expression experiment''' - '''David & Peter''' | ||
| + | |||
<br> | <br> | ||
| - | + | ||
| + | The first expression experiment, testing all the constructs that were made so far. We tested for treated pellet and supernatant, untreated pellets were tested as well. | ||
| + | |||
| + | '''Exression experiment''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Peter & David''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | For this experiment, the following B. subtilis 168 strains were used: | ||
| + | |||
| + | <pre> | ||
| + | |||
| + | |||
| + | |||
| + | </pre> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | All cultures were grown overnight at 37 degrees Celsius in a shaker room, the appropriate antibiotics were used at all points in time during this experiment. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Overnight cultures were used to dilute to a B. subtilis culture of 0,1 OD, these strains were divided into ‘’induced’’ and ‘’non-induced’’. Induction with 0,5% subtilin was done at a OD of 0,5 (approximately 2,5 hours after growth of the 0,1 culture started). | ||
| + | |||
| + | <br> | ||
| + | |||
| + | After that the OD of the cultures was measured every .. hours. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Sample preperation''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | After .. hours, .. after induction, the samples were collected and processed. The following procedures were used: | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Pellet preperation ([[PelletPrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Supernatant processing ([[SupernatantPrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Cell disruption ([[ExtractionCellWallsGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Lysozyme preperation ([[LysozymePrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Analysis was done using SDS-PAGE ([[SDS-PAGEGR]]) and THT staining ([[THTstainingGR]]). | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Results''': | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Growth Curve''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:GrowthCurveGR1.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | '''THT Staining''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:THTGR1.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | '''SDS-PAGE''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:SDS-PAGEGR1.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
<br> | <br> | ||
Revision as of 16:11, 24 October 2010
Expression experiment - David & Peter
The first expression experiment, testing all the constructs that were made so far. We tested for treated pellet and supernatant, untreated pellets were tested as well.
Exression experiment
Peter & David
For this experiment, the following B. subtilis 168 strains were used:
All cultures were grown overnight at 37 degrees Celsius in a shaker room, the appropriate antibiotics were used at all points in time during this experiment.
Overnight cultures were used to dilute to a B. subtilis culture of 0,1 OD, these strains were divided into ‘’induced’’ and ‘’non-induced’’. Induction with 0,5% subtilin was done at a OD of 0,5 (approximately 2,5 hours after growth of the 0,1 culture started).
After that the OD of the cultures was measured every .. hours.
Sample preperation
After .. hours, .. after induction, the samples were collected and processed. The following procedures were used:
Pellet preperation (PelletPrepGR)
Supernatant processing (SupernatantPrepGR)
Cell disruption (ExtractionCellWallsGR)
Lysozyme preperation (LysozymePrepGR)
Analysis was done using SDS-PAGE (SDS-PAGEGR) and THT staining (THTstainingGR).
Results:
Growth Curve
THT Staining
SDS-PAGE
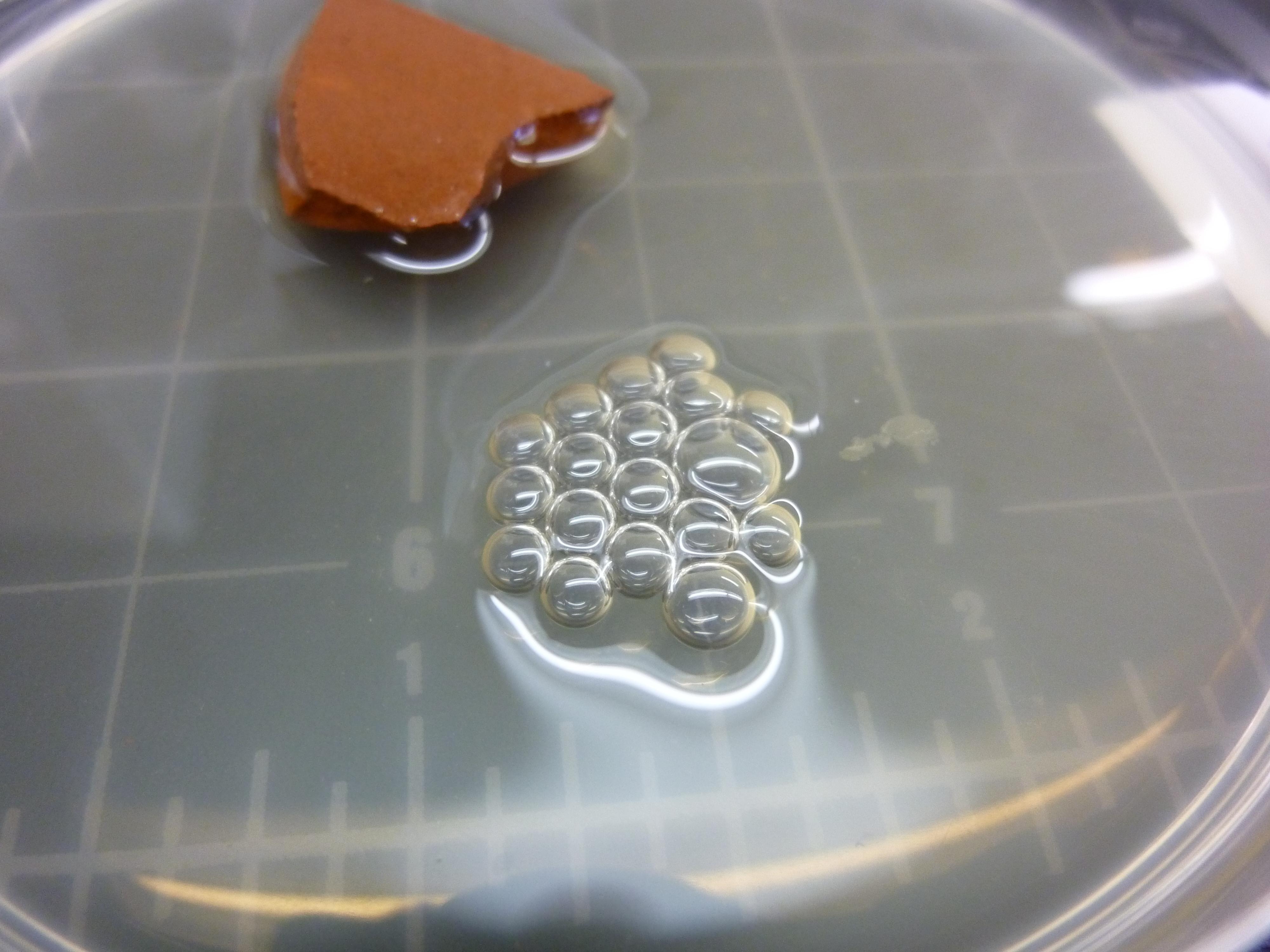
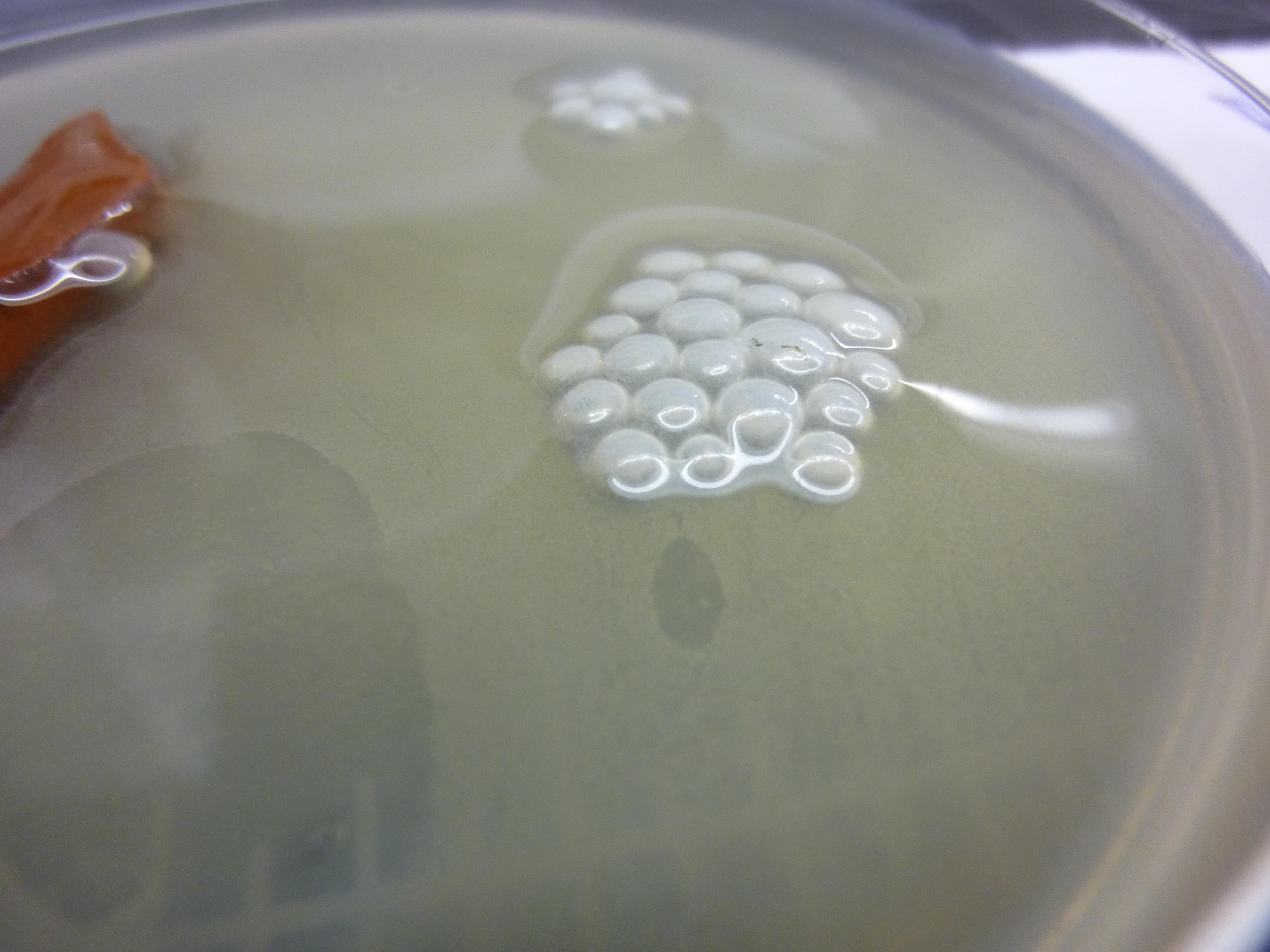
Biofilm formation experiment
Bacillus Subtilis cultures were grown overnight in a Petridish containing 20mL of LB medium and a piece of ceramics to spurr the biofilm formation. The following cultures wehre grown: Bacillus Subtilis, Bacillus Subtilis ΔTasA, Bacillus Subtilis wit chaplin E1, Bacillus Subtilis with chaplin H1, Bacillus Subtilis ΔTasA with chaplin E1, Bacillus Subtilis ΔTasA with chaplin H1
All cultures where inoculated with 100uL of overnight culture. All cultures where run in duplicate, one induced with 1%subtilin, and one without subtilin as a negative control.
We assumed that the ΔTasA would not form a biofilm and the normal phenotype could be restored by the expression of chaplins. Furthermore we assumed that the normal Bacillus Subtilis biofilm would have atered properties.
No such effects could be observed all ΔTasA cultures showed the same non biofilm phenotype
The same goes for all Bacillus Subtilis cultures with TasA, the were all not affected by induction of Chaplins, and showed a normal biofiml forming phenotype.
Our assumption is that either: the expression of chaplins has no visible effect on biofilm formation or, that the subtilin was degraded over night and no proper expression of chaplins took place.
Modellers: Read articles, think about model etc. Joël, Laura, Djoke
 "
"