From 2010.igem.org
(Difference between revisions)
|
|
| Line 25: |
Line 25: |
| | |<b style="font-size:12px">Output Amplification Model</b><br/> | | |<b style="font-size:12px">Output Amplification Model</b><br/> |
| | <ol> | | <ol> |
| - | <li>It was concluded that there is no advantage of 2-step amplification over 1-step amplification. Therefore, the design of a 2-step amplifier was abandoned.</li> | + | <li>It was shown that amplified systems easily outperform the simple production system (control) |
| - | <li>The results concerning the 1-step amplification module were conclusive. It could not be firmly decided whether 1-step amplification is going to perform better than simple production. This is due to the slower start off which is folllowed by a sharp rise to the desirable concentration (in higher active dioxygenase concentrations).</li> | + | <li>It was concluded that there is no advantage of 3-step amplification over 2-step amplification. Therefore, the design of a 3-step amplifier was abandoned.</li> |
| | + | <li>The results concerning the 2-step amplification module were not conclusive. It could not be firmly decided whether 2-step amplification is going to perform better than 1-step amplification. This is because many of the parameters that 2- and 1-step amplifiers are sensitive to could not be determined with certainty.</li> |
| | <li>The conditions for effective amplification were determined.</li> | | <li>The conditions for effective amplification were determined.</li> |
| | </ol> | | </ol> |
| Line 62: |
Line 63: |
| | |<b style="font-size:12px">Output Amplification Model</b><br/> | | |<b style="font-size:12px">Output Amplification Model</b><br/> |
| | Goals: | | Goals: |
| - | <ol><p>This model was mainly developed in order to determine whether simple production is better than 1- or 2-step amplification.</p>Furthermore, an estimation of the speed of the response was desirable. | + | <ol><p>This model was mainly developed in order to determine whether simple production is better than 1-, 2- or 3-step amplification.</p>Furthermore, an estimation of the speed of the response was desirable. |
| | </ol> | | </ol> |
| | Elements of the system: | | Elements of the system: |
Revision as of 21:09, 20 October 2010
| Introduction to modelling
|
In the process of designing our construct two major questions arose which could be answered by computer modelling:
- Output Amplification Model
We came up with an idea of using the amplification of a colour output to make it show within minutes after the stimulus has been added. The question that arose was whether amplification will actually perform better than simple production in the cellular environment. Furthermore, we had trouble deciding whether we should design the amplification module to consist of 1,2 or even more amplification steps. These issues seemed to be difficult enough to employ modelling.
- Surface Protein Model
We came up with a novel idea of detecting organisms that we do not have a specific receptor for. In our particular example, the protease of Schistosoma was meant to cleave a protein displayed on the bacteria's cell wall. The cleaved peptide was supposed to be recognized by the receptor which would activate the colour expression. This solution raised questions about the risk of false positive or whether there are any chances for ComD receptors to be activated in the diluted environment. Modelling of this module would answer these questions.
|
| Results & Conclusions
|
Output Amplification Model
- It was shown that amplified systems easily outperform the simple production system (control)
- It was concluded that there is no advantage of 3-step amplification over 2-step amplification. Therefore, the design of a 3-step amplifier was abandoned.
- The results concerning the 2-step amplification module were not conclusive. It could not be firmly decided whether 2-step amplification is going to perform better than 1-step amplification. This is because many of the parameters that 2- and 1-step amplifiers are sensitive to could not be determined with certainty.
- The conditions for effective amplification were determined.
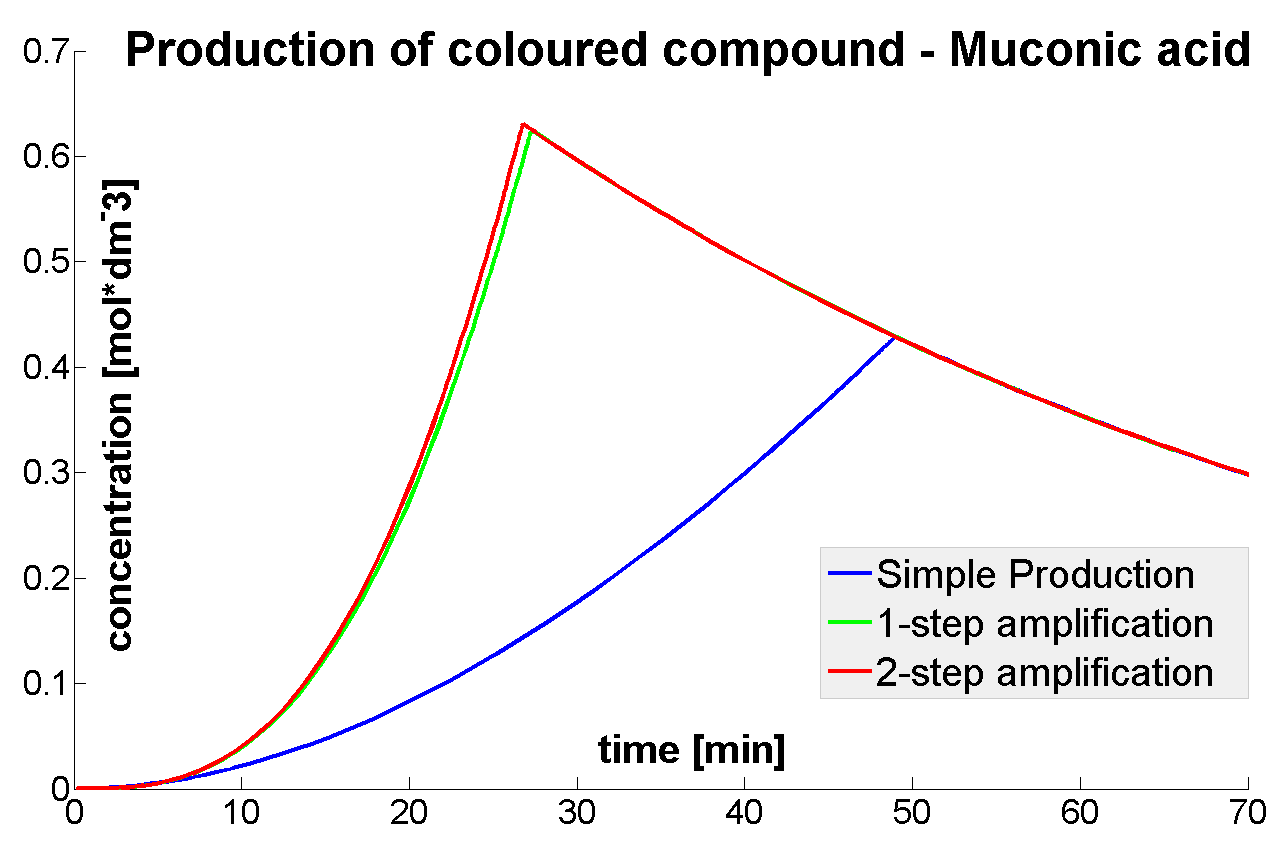
|
| Concentration of coloured compound.
|
Surface Protein Model
- Initial TEV protease concentrations we determined for the optimal activation of the receptor within 1.5 minutes after elastases would have come into contact with our cell.

|
Graph showing when threshold AIP concentration is reached
(for different initial TEV concentrations). Notice log-log scale.
|
|
| Quick overview of models
|
Output Amplification Model
Goals:
This model was mainly developed in order to determine whether simple production is better than 1-, 2- or 3-step amplification. Furthermore, an estimation of the speed of the response was desirable.
Elements of the system:
- Dioxygenase (blue on the diagrams below) is an enzyme that acts on catechol to produce a yellow output. In most of our models dioxygenase was treated as an output because it was found that active dioxygenase acting on catechol produces the coloured output within a split second.
- GFP-Dioxygenase fusion protein (GFP is shown green on the diagrams). Dioxygenase joined by the linker to GFP was assumed to be inactive.
- TEV protease (yellow on the diagrams below) has the ability to cleave the GFP-Dioxygenase fusion protein, hence, it activates dioxygenase
- Split TEV protease (orange on the diagrams below) is an inactive split form of TEV mounted on coiled coils. It can be activated again by coiled coils being cleaved by another active TEV.

|
| Diagram illustrating simple production of dioxygenase.
|
|

|
| Diagram showing 1-step amplification. TEV enzyme is produced (by simple production) to activate pre-produced inactive dioxygenase.
|
|

|
| Diagram illustrating 2-step amplification. Enzyme activates another enzyme which activates dioxygenase. Both pre-products have the same TEV-site, so simply produced TEV is allowed to act directly on dioxygenase too.
|
|
Major assumptions:
- The chemical and enzymatic reactions are modelled according to the Law of Mass Action.
- Our model assumes that the modelled system is inert within the bacterial body or that reactions with other species within the bacterium is negligible. For example, the TEV protease is not supposed to cleave other molecules due to its specifity.
Surface Protein Model
Goals:
The aim of this model is to determine the concentration of Schistosoma elastase or TEV protease that should be added to the bacteria in order to trigger a response. This would allow us to correlate the required concentration for the activation with the concentration of Schistosoma elastase in the lake.<
It was also attempted to model how long it takes for the protease or elastase to cleave the required amount of peptides.
Elements of the system:
- The surface protein consists of a cell wall binding domain, linker, AIP (Auto Inducing Peptide)
- Schistosoma elastase (this is the enzyme released by the parasite) cleaves AIP from the cell wall binding domain at the linker site. In the laboratory we used TEV protease as we could not obtain the Schistosoma elastase.
- The ComD receptor is activated (i.e. AIP concentration is high enough).
Major assumptions:
- The chemical and enzymatic reactions are modelled according to the Law of Mass Action.
- Our model assumes that the modelled system is inert within the bacterial body or that reactions with other species within bacterium is negligible. For example, the TEV protease is not supposed to cleave other molecules due to its specifity.
- Due to our carefully chosen cell concentrations, the diffusion of free AIPs could be neglected. However, this restricts the model to the considered cell concentrations only.
- The threshold for receptor activation was defined by one specific value as opposed to considering intermediate states between fully "off" and "on".
|
 "
"








