Team:Freiburg Bioware/NoteBook/Labjournal/October2
From 2010.igem.org
(→Repetiton: Test digestion of pSB1C3_lITR_phTERT_betaglobin_mGMK_TK30_SDM-PstI_hgH_rITR) |
(→148. labday 13.10.2010) |
||
| Line 1,348: | Line 1,348: | ||
Sequencing comfirmed that the pysical sequence fits our theoretical. Next step will be to remove the remaining PstI restriction site within TK30. | Sequencing comfirmed that the pysical sequence fits our theoretical. Next step will be to remove the remaining PstI restriction site within TK30. | ||
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Repetition of cloning of Rep into Rep78</b></p>==== | ||
| + | <b>Investigator: Kira</b><br/> | ||
| + | '''Comment:''' The last 2 approaches to digest Rep78 and to clone failed because Rep78 was almost gone after PCR purification and was not detectable on the gel, even if I used the double amount of DNA. Thus, new over night culture was grown in order to perform the experiment again <br /> | ||
| + | |||
| + | Regarding the activity conditions of the enzymes, digestion was performed in 2 steps. <br /> | ||
| + | |||
| + | {| border="1" | ||
| + | | align="left" | '''Components''' ||align="left"| <b>Rep78</b> | ||
| + | |- | ||
| + | | align="left" | DNA ||align="left"| 3 µl | ||
| + | |- | ||
| + | | align="left" | BSA (100x) ||align="left"| 2 µl | ||
| + | |- | ||
| + | | align="left" | Buffer no. 3 ||align="left"| 2,0 µl | ||
| + | |- | ||
| + | | align="left" | Enzyme SwaI ||align="left"|1,0 µl | ||
| + | |- | ||
| + | | align="left" | H<sub>2</sub>O ||align="left"| 12 µl | ||
| + | |- | ||
| + | | align="left" | '''Total volume''' ||align="left"| <b>20</b> | ||
| + | |} | ||
| + | <br /> | ||
| + | incubation @ 25 C for approx. 2 h <br /> | ||
| + | Dephosphorylation was performed because of the blunt cleavage. PCR phosphorylation followed in order to get rid off the buffer because Buffer 3 is not appropriate for further digestion. | ||
| + | |||
| + | {| border="1" | ||
| + | | align="left" | '''Components''' ||align="left"| <b>Rep78</b> | ||
| + | |- | ||
| + | | align="left" | DNA ||align="left"| 30 µl | ||
| + | |- | ||
| + | | align="left" | BSA (100x) ||align="left"| 0 µl | ||
| + | |- | ||
| + | | align="left" | Buffer no. 2 ||align="left"| 4,5 µl | ||
| + | |- | ||
| + | | align="left" | Enzyme HindIII ||align="left"|1,0 µl | ||
| + | |- | ||
| + | | align="left" | H<sub>2</sub>O ||align="left"| 9,5 µl | ||
| + | |- | ||
| + | | align="left" | '''Total volume''' ||align="left"| <b>45</b> | ||
| + | |} | ||
| + | <br /> | ||
| + | |||
| + | incubation for 2h at 37C<br /> | ||
| + | |||
| + | [[Image: Rep78.jpg]] | ||
| + | |||
| + | Ligation with T4 ligase <br /> | ||
| + | |||
| + | v(insert)=4,4 ul<br /> | ||
| + | v(vector)= 3,6 ul<br /> | ||
| + | T4 buffer= 1 ul<br /> | ||
| + | T4 ligase= 1 ul<br /> | ||
| + | |||
| + | incubation at RT for 45 min<br /> | ||
| + | |||
| + | Transformation according to the standard protocol with BL21 <br /> | ||
<html> | <html> | ||
</div> | </div> | ||
Revision as of 04:28, 14 October 2010
- March (labday 1)
- April (labday 2 - 5)
- May (labday 6 - 17)
- June (labday 18 - 45)
- July (labday 46 - 75)
- August part 1 (labday 76 - 92)
- August part 2 (labday 93 - 106)
- September part 1 (labday 107 - 123)
- September part 2 (labday 124 - 135)
- October part 1 (labday 136 - 145 )
- October part 2 (labday 146 - 155 )
- October part 3 (labday 156 - 166 )
- November (labday 167 - 170 )
- Cellculture
146. labday 11.10.2010
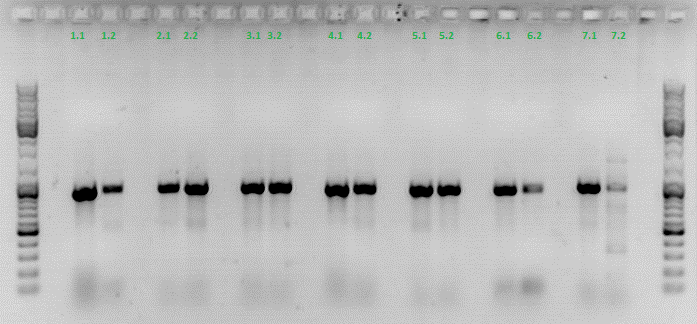
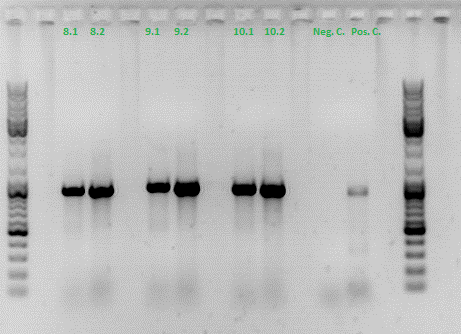
Colony PCR of Cloning VP2 Fusion and Super constructs into pSB1C3
Investigator: Achim, Hanna
Comment: Because yesterday's cloning didn't deliver a good separartion of the expected gel bands. Nevertheless ligation and trafo was performed. In order to immediately find out, whether we received successful results a colony PCR will be performed.
Two clones were picked from each plat. In addition to that a positive (pAAV_RC) and a negative control (pSB1C3_lITR) was prepared.
Used primer: 4200 rev and Cap3500 for. Expected fragment size: 885 bp.
PCR was performed following the standard protocol.
All samples match the positive control!
To do: Mini-Prep and sequencing.
Preparation of SDS-PAGE gel (10%)
Investigator: Hanna
Comment: In order to perform a Western Blot of different virus capsids (with and without capsid-motifs), 2 10% SDS polyacrylamid gels were prepared.
Resolving gel: 15 mL
- H2O: 5.9 mL
- Acryl-bisacrylamide mix (30%): 5 mL
- Tris (1.5 M, pH 8.8): 3.8 mL
- SDS (10%): 0.15 mL
- Ammonium persulfate (10%): 0.15 mL
- TEMED: 0.006 mL
Stacking gel (5%): 5 mL
- H2O: 3.4 mL
- Acryl-bisacrylamide mix (30%): 0.83 mL
- Tris (1.5 M, pH 6.8): 0.63 mL
- SDS (10%): 0.05 mL
- Ammonium persulfate (10%): 0.05 mL
- TEMED: 0.005 mL
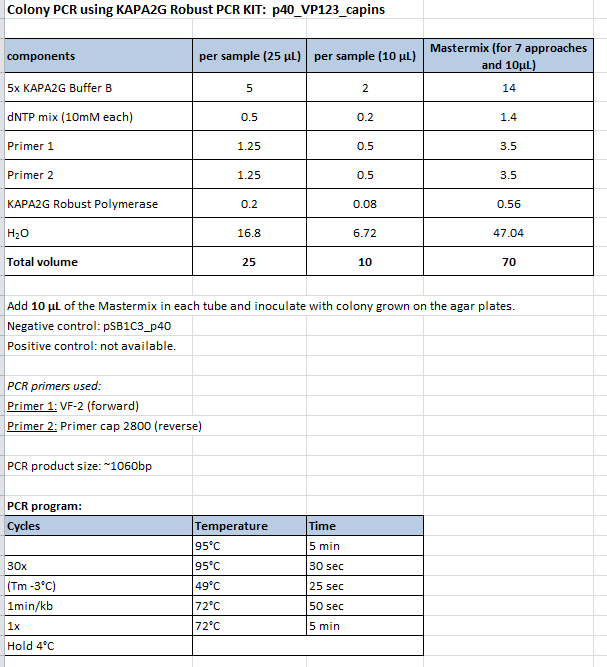
Colony PCR of p40_VP123_capins
Investigator: Bea
Comment: Since the first attempt did not work,and no cells grew on the plate and the same ligation was transformed again into BL-21 and a lot of clones grew on the plate, I decided to perform a colony PCR in order to check several colonies and to inoculate at the same day for a Midi-Prep.
Protocol:
- Primer used: O162
- Primer used: O38
The PCR products were loaded on a 1% agarose gel. The results can be seen above in the gel picture:
Result: We can see two things: The cloning of p40 to VP123 worked quiet well AND the Robust PCR Kit which was used for the first time worked as well.
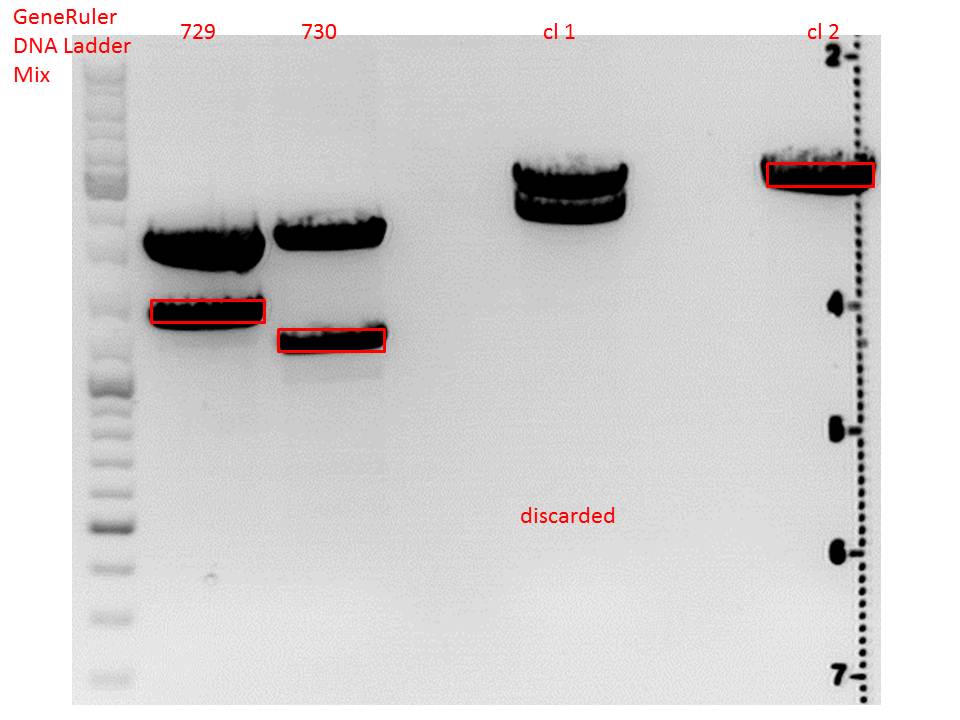
Cloning of lITR_CMV_betaglobin and lITR_phTERT_betaglobin into pSB1C3_CD
Investigator: Stefan
Comment: To produce another GOI for testing in cell culture, the cytosine deaminase needs to be assembled with lITR_promotor_betaglobin. In the next step hgH_rITR needs to be added.
Vector name:
pSB1C3_CD clone 1
pSB1C3_CD clone 2
Insert name:
pSB1C3_lITR_CMV_beta-globin (P729)
pSB1C3_lITR_phTERT_beta-globin (P730)
Digestion:
| components | volume CD clone 1 + 2 /µl | volume P729 /µl | volume P730 /µl |
| DNA | 6 | 14 | 6 |
| BSA (10x) | 2 | 2 | 2 |
| Buffer 4 (10x) | 2 | 2 | 2 |
| Enzyme EcoI | 1 | 1 | 1 |
| Enzyme XbaI | 1 | - | - |
| Enzyme SpeI | - | 1 | 1 |
| H2O | 8 | - | 8 |
| Total volume (e.g. 15,20,25,30 µl) | 20 | 20 | 20 |
Gel:
0,5 g Agarose, 50 ml TAE (1%), 3 µl GELRED , at 115 Volt
CD clone 1 yielded to bands around 2500 bp to 3000 bp. Since the vector was cut only using EcoRI and SpeI, it was expected to be linearized, not to be cut into two fragments this size. Therefore, this sample was discarded and cloning was continued using CD clone 2.
Gel extraction:
Was performed according to protocol.
T4 Ligation:
| ligation name | 729 + CD cl2 | 730 + CD cl2 |
| volume of vector | 3,67 | 2,82 |
| volume of insert | 4,33 | 5,18 |
| T4 ligase buffer (10x) | 1 | 1 |
| T4 ligase | 1 | 1 |
Transformation:
Was performed according to standard protocol using BL21 cells.
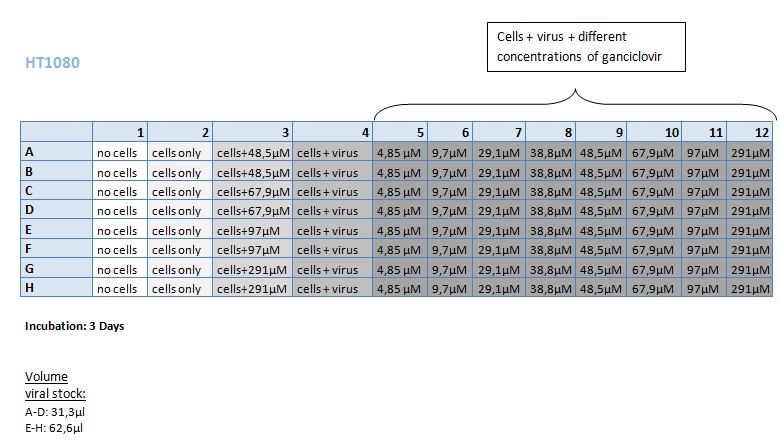
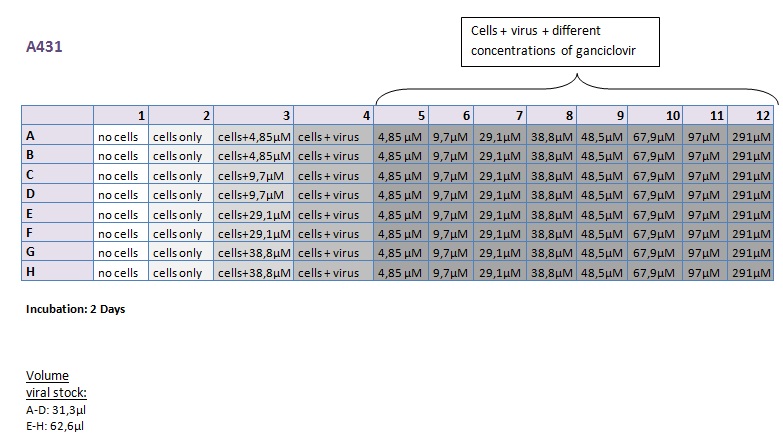
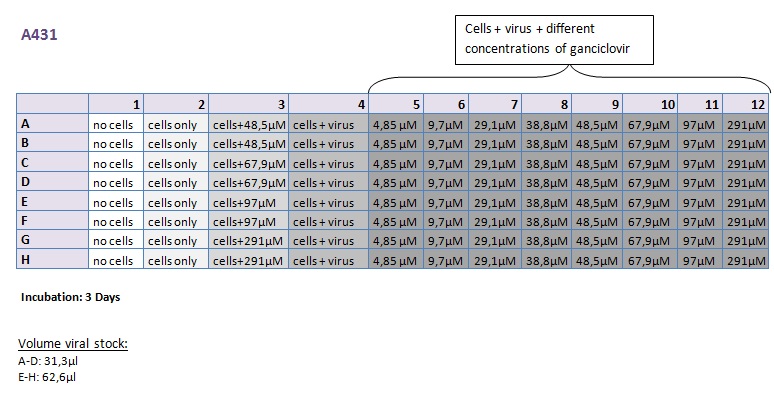
Seeding HT1080 and A431 for testing different concentrations of ganciclovir by MTT-Assay
Investigator: Kerstin, Anissa
- Seeding 4x 96-well plates: 2x HT1080 and 2x A431
FACS-Analysis
Investigator: Kerstin
...
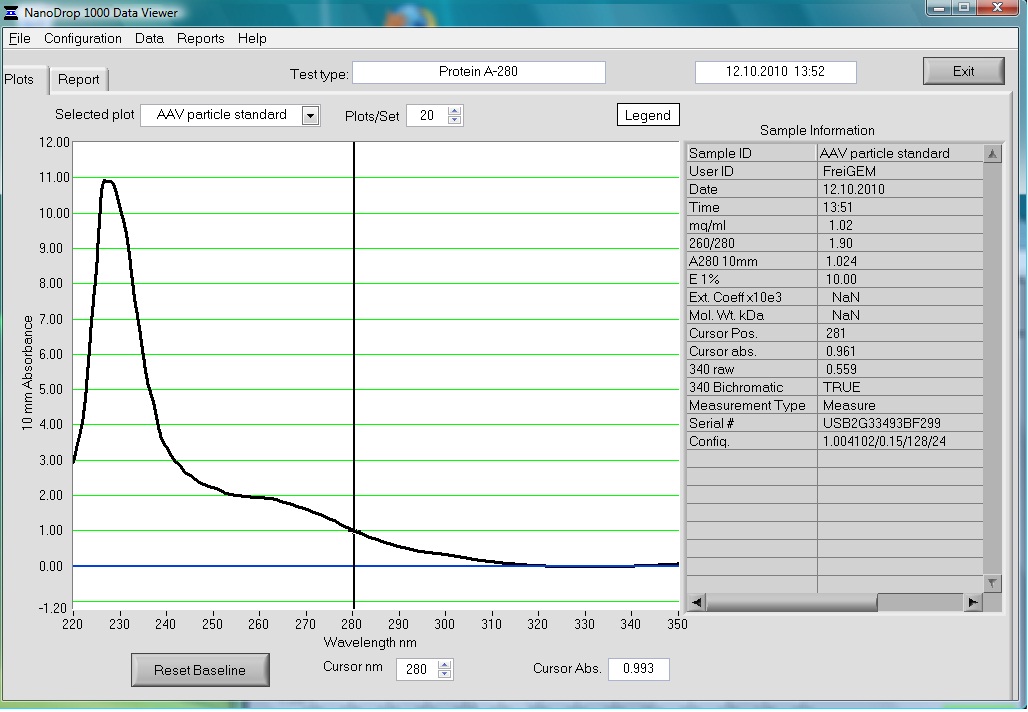
Preparation of the ELISA
Investigator: Volker
The AAV particle standard that contains 3.6x10^9 viral particles was dissolved in 500µl as described in the protocol of the Progen AAV Titration ELISA and a absorption spectrum was measured.
These purified and concentrated viral particles could be used for biophysical measurements, there for the possibility to detect the viral particles by absorption was interesting for us.
The Spectrum measured in the NanoDrop is the following:
147. labday 12.10.2010
Sequencing analysis: pSB1C3_mGMK_TK30 and pSB1C3_CD
Investigator: Stefan
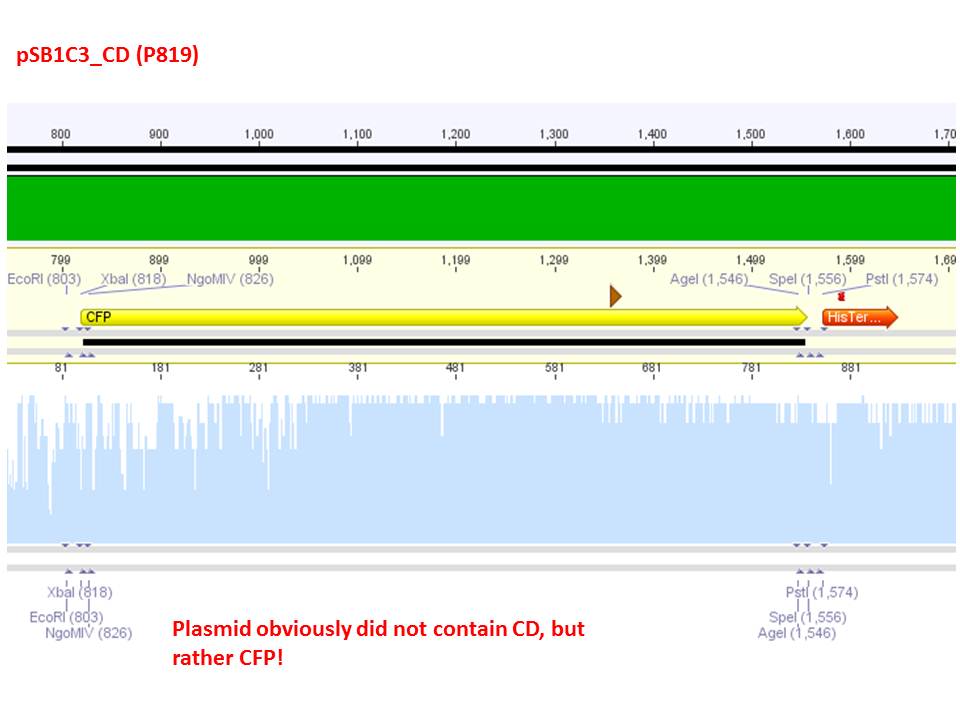
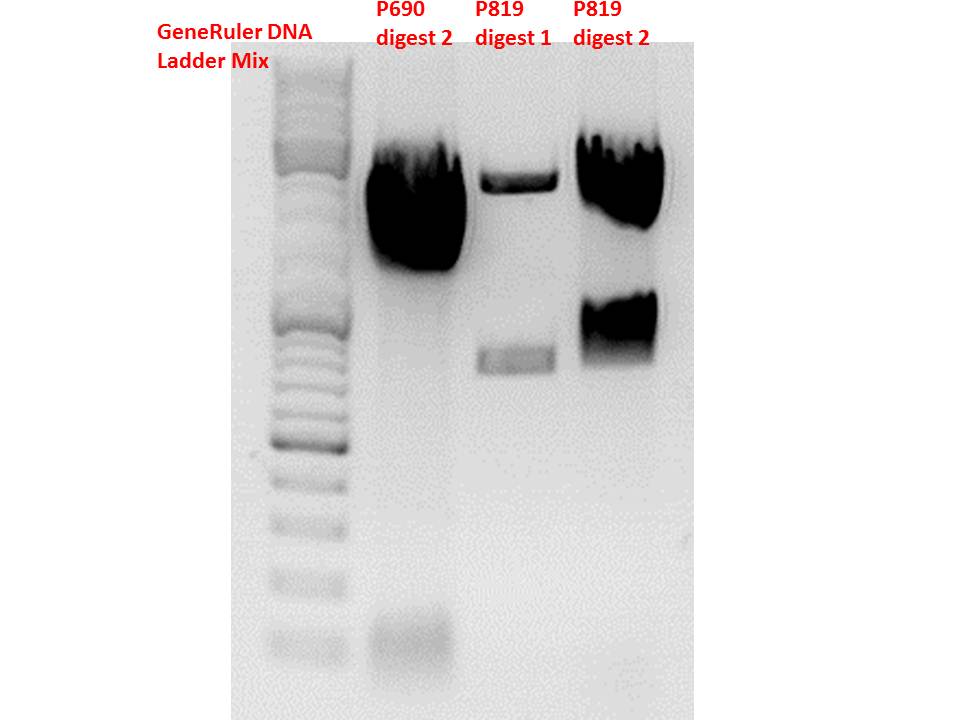
Test digestion of pSB1C3_CD and pSB1C3_CD_SDM-PstI_new
Investigator: Stefan
Comment: Nachricht von Stefan an Stefan: bitte ergänzen!
Comment: Sequencing results revealed that there is CFP rather than CD in P819. In order to verify this and exclude the possibility that the wrong plasmid was sent for sequencing test digestions were performed. Additionally the original vector at which the SDM to remove the PstI was performed was digested as well to verify its insert.
| Components | digest 1 / µl | digest 2 / µl |
| DNA | 1 | 7 |
| Buffer 4 | 1 | 1 |
| BSA (10x) | 1 | 1 |
| Enzyme I | SpeI 0,3 | PstI 0,3 |
| Enzyme II | XbaI 0,3 | XbaI 0,3 |
| H2O | 6,4 | 0,4 |
| Total volume | 10 | 10 |
Comment: Using these digestion approaches, it was expected for CFP to deliver always bands at ~750 bp. For the CD it was expected to show bands at 90 bp, 1200 bp and 2000 bp if the CD still contains the PstI restictiton site, 1300 bp and 2000 bp if it was removed.
Gel:
0,5g agarose, 50 ml TAE (1%), 3 µl GELRED, 115 Volt, running time ~50 minutes
Comment: As it can be seen, P819 does contain CFP, therefore a mistakenly sequenced plasmid can be excluded. Also it can be seen that P690 contains the CD. Therefore an additonal SDM of P690 to remove the PstI restriction site is necessary.
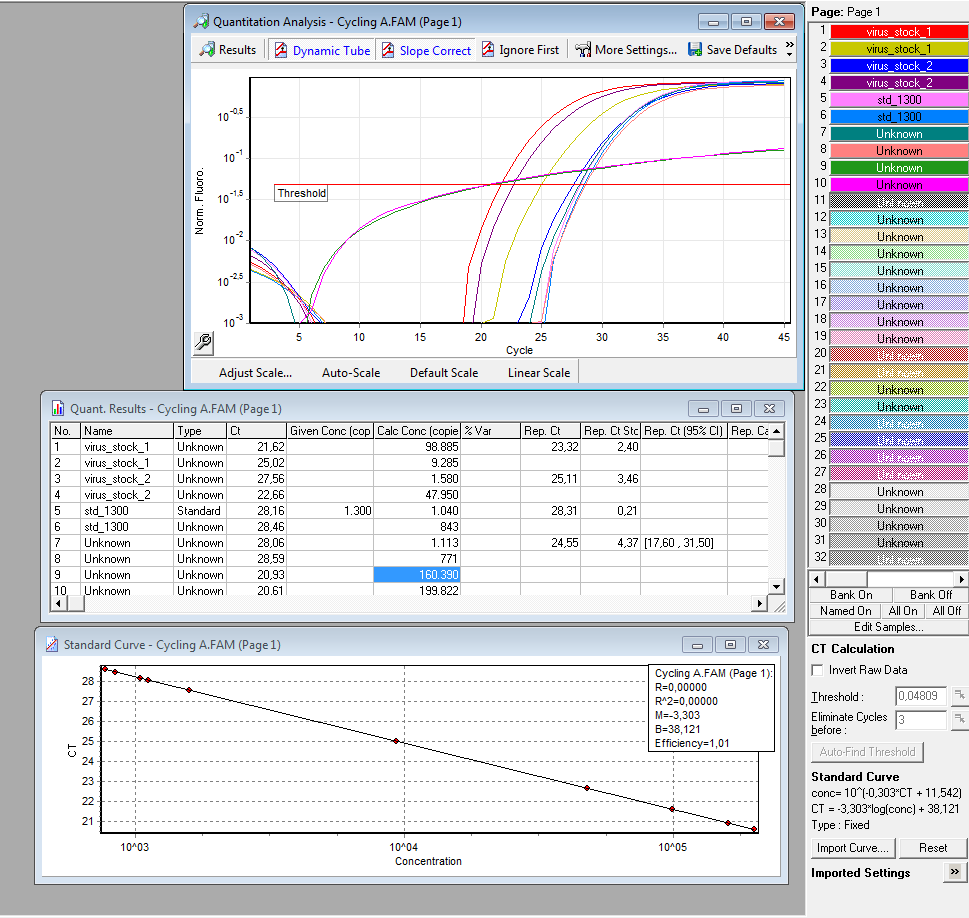
qPCR of virus stock: P816/ TKGMk-Standard; R/C: P468 from 5.10. (VLP harvested by Adrian)
Investigator: Achim
Protocol for quantitative real-time PCR of virus particles
Date: 12.10.
Achim
Virus Stock: A.F.; P816; TKGMK-Standard; R/C: P468; 5.10; consists of supernatant from pelleted cell fragments.
Following the Protocol used by (Rohr et al., 2002).
I digested 5 µl virus dilution with 7,5 µl DNAse I (7,5 units) and 25 µl 50 mM MgCl2 (end concentration 25 mM) in a final volume of 50 µl at 37°C for 30 min.
è DNAse should be heat inactivated at 65°C for 10 min. I forgot that step. The enzyme should however be inactivated in the initial denaturation step.
I prepared the following PCR reactions:
|
Sample |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
9 |
10 |
|
|
1.1 |
1.2 |
2.1 |
2.2 |
S.1 |
S.2 |
NP1 |
NP2 |
MM for samples 1 -8 |
NH1 |
NH2 |
|
PCRmix |
12.5 |
12.5 |
12.5 |
12.5 |
12.5 |
12.5 |
12.5 |
12.5 |
112.5 |
12.5 |
12.5 |
|
Primer for |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
9 |
- |
- |
|
Primer rev |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
9 |
- |
- |
|
Template |
2 |
2 |
2 |
2 |
5 |
5 |
- |
- |
- |
- |
- |
|
H2O |
8.5 |
8.5 |
8.5 |
8.5 |
5.5 |
5.5 |
10.5 |
10.5 |
- |
12.5 |
12.5 |
|
Total |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
25 |
25 |
|
Step |
Time |
Temp |
|
Initial denaturation |
7’ |
95°C |
|
|
|
|
|
Denaturation |
10’’ |
95°C |
|
Annealing/Extension |
30’’ |
60°C |
|
Number of Cycles |
45 |
|
Results:
è Both negative controls containing primers were contaminated with DNA.
è Negative controls containing only H2O were negative.
è Values for plasmids/reaction vary; from 1 – 100 for the same virus stock
è Dilution of the virus solution: 1:10; 2µl PCR sample: 3.2 * 10^5 virus particles/ml
è Because of the contaminated negative controls and the high deviation, I will repeat the experiment tomorrow to verify the results.
References:
Rohr, U., Wulf, M., Stahn, S., Steidl, U., Haas, R., Kronenwett, R., et al. (2002). Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. Journal of virological methods, 106(1), 81-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12367732.
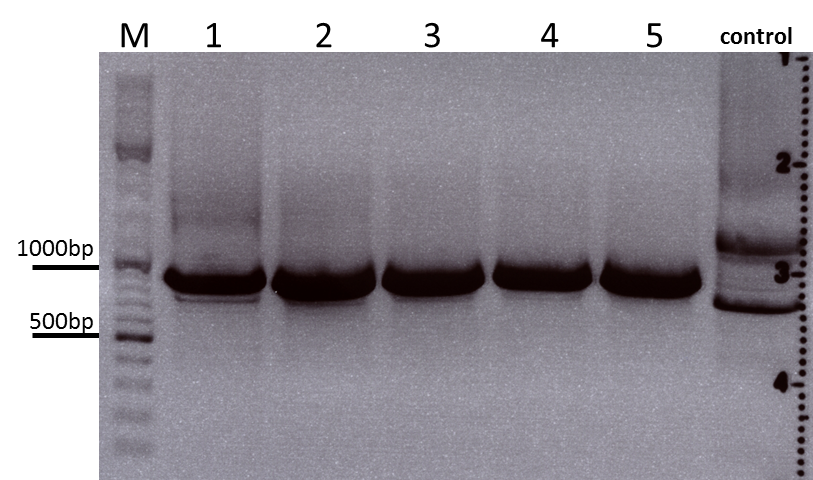
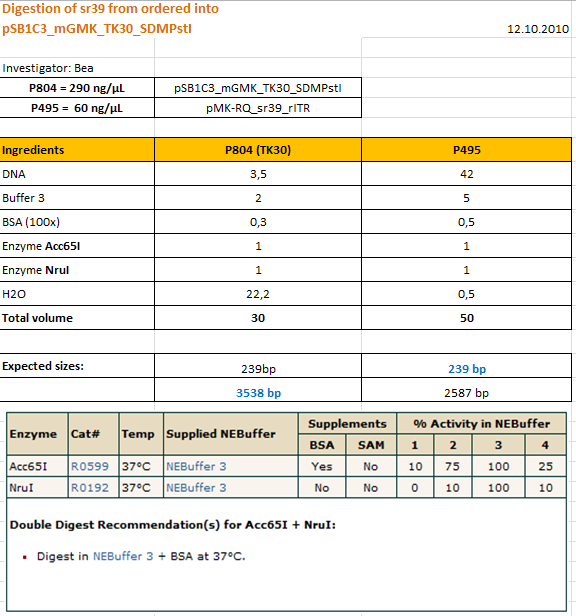
Cloning sr39 into mGMK_TK30
Investigator: Bea
Comment: We do not only want to submit the tk30, a mutant version of the thymidine kinase, but as well another mutant protein which is named sr39. SR39 seems to have a better activity than the tk30 without being fused to the guanylate kinase (mgmk), but the sr39 also works quite well as a fusion protein. We ordered one part of the sr39 and we are now subcloning it into the mgmnk_tk30 construct in order to obtain mgmk_sr39.
Protocol:
The digested fragments were loaded on a 1.2% agarose gel. The results can be seen above in the gel picture: I cut out the small visible band in the right lane, and the upper intensive band of the left lane.
Result:
After gel extraction has been performed, I ligated the two fragments and transformed BL-21 cells, plated them on cm agar plates and incubated them over night at 37°C.
Concentration of virus stock for Western Blot analysis
Investigator: Hanna
For the first Western Blot try, we investigate whether is is enough to concentrate the virus stock (from cell supernatant) and then to simply load it onto a SDS-PAGE gel and perfom immuno-staining.
For this purpose 8 mL virus stock (pHelper, pAAV_RC, GOI=YFP) were concentrated via amicon filtration.
- First, amicon filter was washed with 4 mL PBS.
- Then 4 mL virus stock was loaded onto the filter and centrifuged at 3000 rpm for 15 minutes.
- Flow-through was discarded and additional virus stock was loaded, centrifuged at 3000 rpm for 25 minutes.
- Flow-through was discarded, remaining solution was re-suspended and additional virus stock was added. Amicon filter was centrifuged at 3000 rpm for 50 minutes.
- Flow-through was discarded. Residual protein solution: 500 µL. Filter was washed with 3 mL PBS, centrifuging 35 minutes two times.
- 2 x 40 µL protein solution (16x) was taken and mixed with Laemmli buffer and stored over night @ 4°C.
To do: SDS-PAGE and Western Blot tomorrow.
Perfomance of another try by harvesting viruses of the cell pellet with RIPA buffer & freeze/ thaw.
Western Blot of unmodified, and modified capsids (N-terminal fusion and VP1 insertion with CFP ref. mVenus) --> size shift should be detectable.
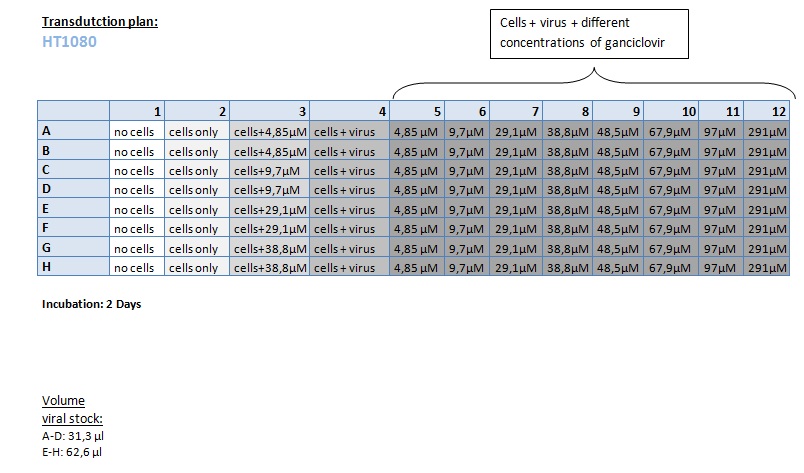
Transduction of HT1080 and A431
Investigator: Kerstin
Mini-Prep and test digestion of several constructs
Investigator: Stefan
Glycerol stocks were prepared:
- B651 = pSB1C3_P40_VP123
- B652 = pSB1C3_lITR_phTERT_ß-globin_mGMK_TK30_pSTI_SDM_hgH_rITR
- B653 = pSB1C3_lITR_phTERT_ß-globin_mGMK_TK30_pSTI_SDM_hgH_rITR
- B654 = pSB1C3_lITR_CMV_ß-globin_mGMK_TK30_hgH_rITR
- B655 = pSB1C3_lITR_CMV_ß-globin_mGMK_TK30_hgH_rITR
- B656 = pSB1C3_CMV_VP123_453_Z34C_HSPG-KO
- B657 = pSB1C3_CMV_VP123_453_Z34C_HSPG-KO
- B658 = pSB1C3_CMV_VP123_453_RGD_HSPG-KO
- B659 = pSB1C3_CMV_VP123_453_RGD_HSPG-KO
- B660 = pSB1C3_lITR_phTERT_ß-globin_CFP_hgH-rITR
- B661 = pSB1C3_lITR_phTERT_ß-globin_CFP_hgH-rITR
Mini-Prep was performed according to standard protocol:
- P821 = pSB1C3_P40_VP123 c = 327,4 ng/µl
- P822 = pSB1C3_lITR_phTERT_ß-globin_mGMK_TK30_pSTI_SDM_hgH_rITR c = 342,9 ng/µl
- P823 = pSB1C3_lITR_phTERT_ß-globin_mGMK_TK30_pSTI_SDM_hgH_rITR c = 324,0 ng/µl
- P824 = pSB1C3_lITR_CMV_ß-globin_mGMK_TK30_hgH_rITR c = 81,6 ng/µl
- P825 = pSB1C3_lITR_CMV_ß-globin_mGMK_TK30_hgH_rITR c = 148,4 ng/µl
- P826 = pSB1C3_CMV_VP123_453_Z34C_HSPG-KO c = 105,8 ng/µl
- P827 = pSB1C3_CMV_VP123_453_Z34C_HSPG-KO c = 121,1 ng/µl
- P828 = pSB1C3_CMV_VP123_453_RGD_HSPG-KO c = 188,4 ng/µl
- P829 = pSB1C3_CMV_VP123_453_RGD_HSPG-KO c = 140,4 ng/µl
- P830 = pSB1C3_lITR_phTERT_ß-globin_CFP_hgH-rITR c = 197,2 ng/µl
- P831 = pSB1C3_lITR_phTERT_ß-globin_CFP_hgH-rITR c = 207,3 ng/µl
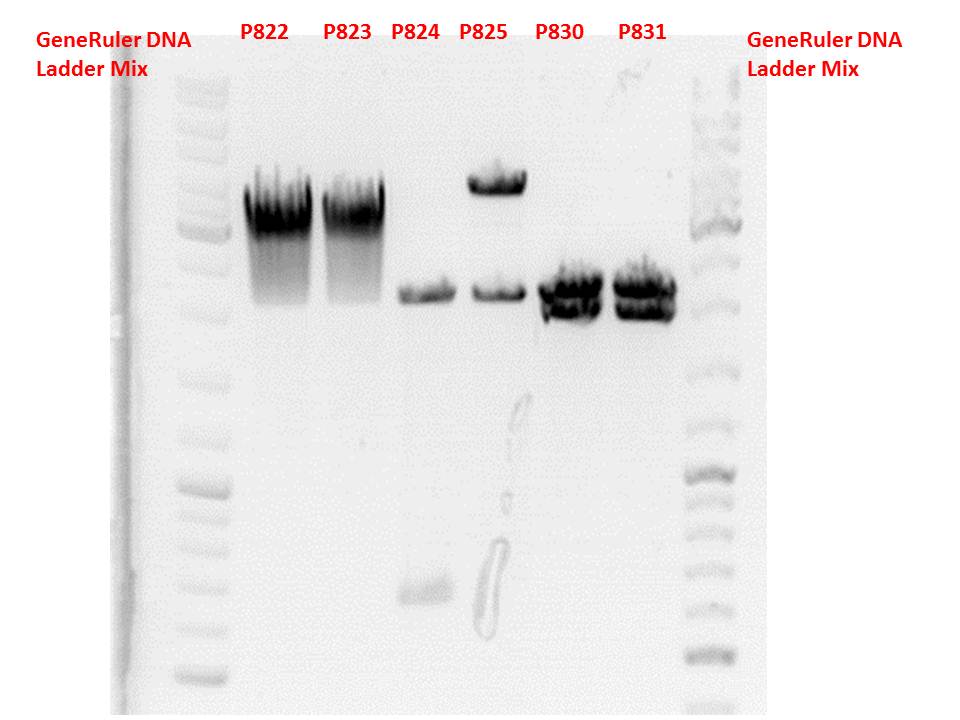
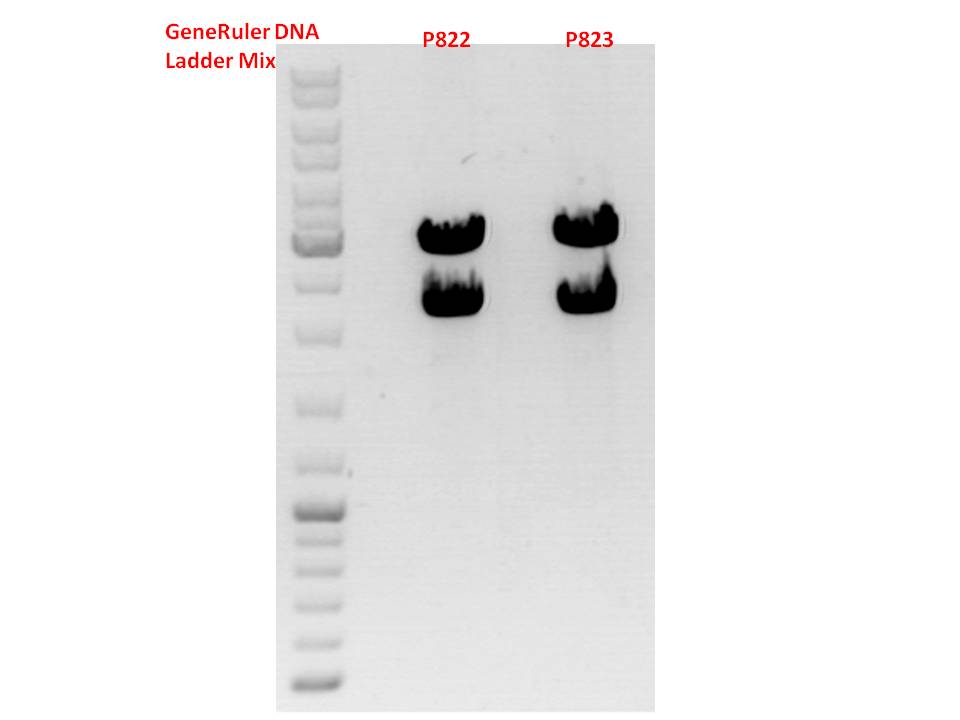
Test digestion:
| Components | P822 + P823 / µl | P824 + P825 / µl | P830 + P831 / µl |
| DNA | 1,5 | 1,5 | 2 |
| Buffer 4 | 1 | 1 | 1 |
| BSA (10x) | 1 | 1 | 1 |
| PstI | 0,3 | - | - |
| XbaI | 0,3 | 0,3 | 0,3 |
| SpeI | - | 0,3 | 0,3 |
| H2O | 5,9 | 5,9 | 5,4 |
| Total volume | 10 | 10 | 10 |
Gel:
0,5g agarose, 50 ml TAE (1%), 3 µl GELRED, 115 Volt, running time ~50 minutes
Comment: P822 and P823 smear a lot, therefore evaluation is difficult. Another test digestion would be reasonable.
P824 contains hgH_rITR, therefore it will be discarded. P825 looks well and was sent for sequencing.
P830 and P831 look well and will be sent for sequencing tomorrow.
Repetiton: Site-directed mutagenesis in pSB1C3_CD construct
Investigator: Stefan
Comment: Sequencing revealed that there is no CD in our pSB1C3_CD but rather CFP. So, anonther approach of the SDM needs to be performed.
Protocol using the QuickChange Lightning Kit from Stratagene:
Ingredients:
| Ingredients | Volume / µl |
| 10x reaction buffer | 2,5 |
| dNTP | 0,5 |
| forward primer: O191 | 0,56 |
| reverse primer: O192 | 0,57 |
| DNA Template | 0,5 (1:10 dilution) |
| QuikSolution Reagent | 0,75 |
| QuikChangeLightning Enzyme | 0,5 |
| H2O | 14,35 |
| Total volume | 25 |
The following PCR program was used:
- 95 °C 120 s
- 95 °C 20 s
- 60 °C 60 s
- 68 °C 105 s
- Goto step 2 repeat 17 times
- 68 °C 300 s
Digestion using DpnI:
To digest methylated and hemi-methylated DNA, 1 µl of DpnI was added and the mixture was incubated for 10 minutes at 37 °C.
Transformation:
Trafo was performed according to protocol, but using XL1b cells instead of XL-10 Gold cells.
148. labday 13.10.2010
SDS Page of unmodified AAV2 capsids
Investigator: Hanna
Comment: Yesterday a 8x concentration of AAV2 supernatant-stock was performed. 10 µL Laemmli-buffer was added to 40 µL AAV stock (8x) and stored over night at 4°C.
Today a SDS-PAGE of these samples will be performed.
Samples were incubated for 10 minutes at 95°C for denaturation.
Loading plan:
| 5 µL PageRuler Prestained Protein Ladder | 10 µL Sample | 15 µL Sample | 25 µL Sample |
After running front reached the end of the gel, PAGE was stopped.
Western Blot:
Three layers of transfer buffer soaked whatman paper were placed onto the blotter. A polyvinylidene difluoride membrane was activated by methanol (5 seconds) and then incubated for 5 minutes in transfer buffer. Membrane was then placed onto the whatman paper, gel was carefully layed on top. Air bubbles were removed, ensuring elictricity flow. After coverage by 3 further layers of whatman, blotting was performed at 200 mA for 1 hour.
Blocking:
In order to minimize nonspecific antibody interactions, membrane was incubated in blocking buffer (TBS (pH 7.5), 5% milk powder) for one hour, shaking.
Primary antibody incubation:
A 1:10 dilution of the B1 antibody in TBS + 1% milk was prepared. Membrane was incubated in 50 mL falcon over night at 4°C, rotating.
Midi-Prep
Investigator: Chris W.
Midi-Prep of:
pSB1C3_CMV_VP123_453_Z34C =P832 =B656
pSB1C3_CMV_VP123_453_RGD_HSPG-KO =P833 =B658
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 1 =P834 =B561
pSB1C3_001_RC_IRCK_P5tataless clone 1 =P835 =B516
pSB1C3_lITR_CMV_betaglobin_mVenus_hGH_rITR clone1 =P836 =B200
pSB1C3_lITR_CMV_betaglobin_mVenus_hGH_rITR clone1 =P837 =B200
The Midi-Preps were performed according to the standard protocol yielding the following concentrations:
| plasmid-no. | P832 | P833 | P834 | P835 | P836 | P837 |
| concentration (ng/µl) | 3615,8 | 1504,7 | x | x | x | x |
Comment: P834-837 given to sven
Repetiton: Test digestion of pSB1C3_lITR_phTERT_betaglobin_mGMK_TK30_SDM-PstI_hgH_rITR
Investigator: Stefan
Comment: Repetiton of yesterday's test digestion which yielded no clear band at 2000 bp as it was expected.
| Components | P822/P823 / µl |
| DNA | 1,5 |
| Buffer 4 | 1 |
| BSA (10x) | 1 |
| SpeI | 0,3 |
| XbaI | 0,3 |
| H2O | 5,9 |
| Total volume | 10 |
Gel:
0,45g agarose, 50 ml TAE (0,9%), 3 µl GELRED, 90 Volt, running time ~110 minutes
Comment: This time both samples look well. P822 will be sent for sequencing.
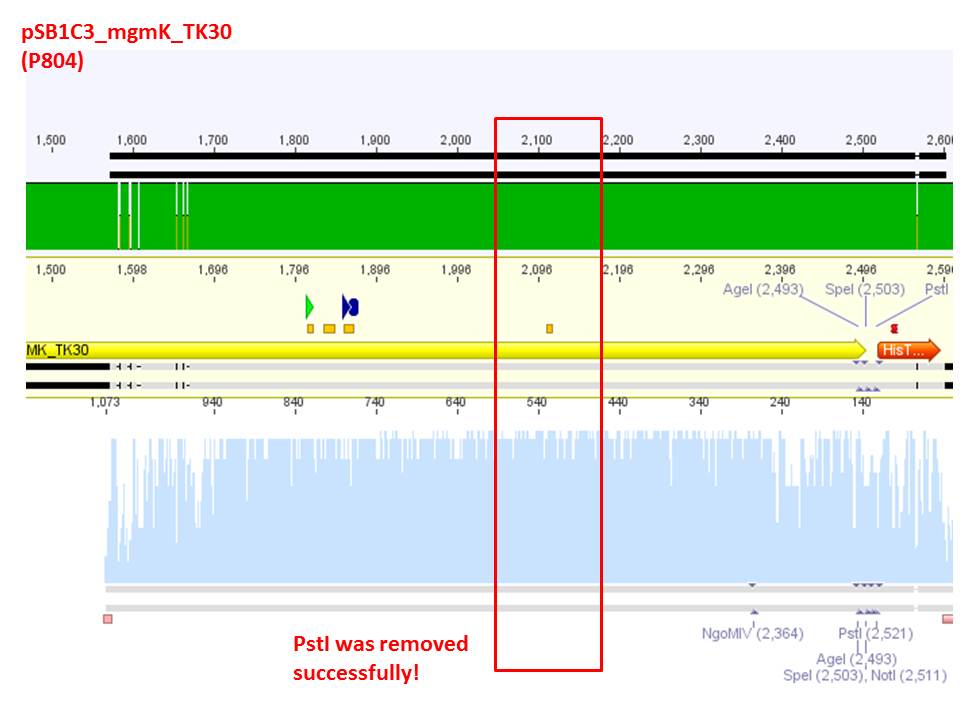
Sequencing analysis: pSB1C3_leftITR_pCMV_betaglobin_mGMK_TK30_hgH_rITR (P825)
Investigator: Stefan
For sequencing the following primers were used_
- qPCR primer CMV for (O20)
- GOI primer for (O30)
- seq. primer TK GMK for (O23)
- TK30 SDM Pst for (O191)
Sequencing comfirmed that the pysical sequence fits our theoretical. Next step will be to remove the remaining PstI restriction site within TK30.
Repetition of cloning of Rep into Rep78
Investigator: Kira
Comment: The last 2 approaches to digest Rep78 and to clone failed because Rep78 was almost gone after PCR purification and was not detectable on the gel, even if I used the double amount of DNA. Thus, new over night culture was grown in order to perform the experiment again
Regarding the activity conditions of the enzymes, digestion was performed in 2 steps.
| Components | Rep78 |
| DNA | 3 µl |
| BSA (100x) | 2 µl |
| Buffer no. 3 | 2,0 µl |
| Enzyme SwaI | 1,0 µl |
| H2O | 12 µl |
| Total volume | 20 |
incubation @ 25 C for approx. 2 h
Dephosphorylation was performed because of the blunt cleavage. PCR phosphorylation followed in order to get rid off the buffer because Buffer 3 is not appropriate for further digestion.
| Components | Rep78 |
| DNA | 30 µl |
| BSA (100x) | 0 µl |
| Buffer no. 2 | 4,5 µl |
| Enzyme HindIII | 1,0 µl |
| H2O | 9,5 µl |
| Total volume | 45 |
incubation for 2h at 37C
Ligation with T4 ligase
v(insert)=4,4 ul
v(vector)= 3,6 ul
T4 buffer= 1 ul
T4 ligase= 1 ul
incubation at RT for 45 min
Transformation according to the standard protocol with BL21
149. labday 14.10.2010
150. labday 15.10.2010
151. labday 16.10.2010
152. labday 17.10.2010
153. labday 18.10.2010
154. labday 19.10.2010
155. labday 20.10.2010
 "
"