Team:Washington/Gram Negative/Test
From 2010.igem.org
| Line 31: | Line 31: | ||
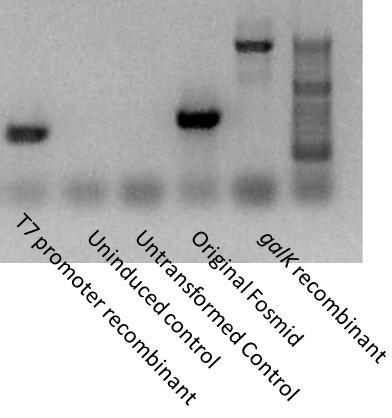
[[Image:Washington T7-PCR.jpg|200px|thumb|right|T7 recombinant cassette insertion]] | [[Image:Washington T7-PCR.jpg|200px|thumb|right|T7 recombinant cassette insertion]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
=SDS-PAGE Protein Array= | =SDS-PAGE Protein Array= | ||
Revision as of 04:15, 9 October 2010
Western blotting for proper Tse2 expression
In order to determine that Tse2 and Tsi2 were only being produced in the presence of 3OC6HSL, E. coli MG1655 containing the F2620-Tse2-Tsi2 construct was cultured in liquid LB containing either 10,000nM 3OC6HSL, or no HSL. the cultures were pelleted, and western blotted for Tse2. The cultures grown in 3OC6HSL+ media showed bands on the western blot (figure x) indicative of Tse2 being produced when 3OC6HSl is present. The cultures grown in media without 3OC6HSL showed no bands ( figure X), meaning that Tse2 is not being produced unless HSL is present. This is exactly the behavior that was expected if the Tse2/tsi2 system was working properly. The survival of cells in the HSL+ media despite the production of Tse2, combined with the sequence confirmation of Tse2 in the construct implies that tsi2 is working as an antitoxin.

SDS-PAGE Protein Array
Fha1 (Forkhead-associated protein) is an essential component of the Type VI Secretion System. We confirmed the utility of our promoter insertion by using an SDS-PAGE assay with anti-Fha antibody to probe for expressed protein. The recombinant fosmid was transformed into a T7 expression strain, BL21(DE3), which produces T7 RNA polymerase in the presence of IPTG. The Fha1 protein was expressed under induced conditions.
 "
"