Team:Freiburg Bioware/NoteBook/Labjournal/October
From 2010.igem.org
(→140. labday 05.10.2010) |
(→Site-directed mutagenesis in pSB1C3_mGMK_TK30 construct) |
||
| Line 805: | Line 805: | ||
===<p style="font-size:17px; background-color:#00dd77;">140. labday 05.10.2010</p>=== | ===<p style="font-size:17px; background-color:#00dd77;">140. labday 05.10.2010</p>=== | ||
====<p style="font-size:18px; background-color:#66bbFF;"><b>Site-directed mutagenesis in pSB1C3_mGMK_TK30 construct</b></p>==== | ====<p style="font-size:18px; background-color:#66bbFF;"><b>Site-directed mutagenesis in pSB1C3_mGMK_TK30 construct</b></p>==== | ||
| + | <b>Investigator: Bea</b> | ||
| + | <br/> | ||
| + | <p style="color:#66bbff;"><i>Comment</i>: Since we are still waiting for the ordered TK30, idea was to mutate the PstI site in the TK30 region. After mutating the PstI it is ready for submitting. Addtionally the ordered and received sr3ß can be subcloned. </p> | ||
====<p style="font-size:18px; background-color:#66bbFF;"><b>Cellculture: Bad news</b></p>==== | ====<p style="font-size:18px; background-color:#66bbFF;"><b>Cellculture: Bad news</b></p>==== | ||
Revision as of 21:58, 5 October 2010
Team:Freiburg Bioware/SubNoteBook
- March (labday 1)
- April (labday 2 - 5)
- May (labday 6 - 17)
- June (labday 18 - 45)
- July (labday 46 - 75)
- August part 1 (labday 76 - 92)
- August part 2 (labday 93 - 106)
- September part 1 (labday 107 - 123)
- September part 2 (labday 124 - 135)
- October part 1 (labday 136 - 145 )
- October part 2 (labday 146 - 155 )
- October part 3 (labday 156 - 166 )
- November (labday 167 - 170 )
136. labday 01.10.2010
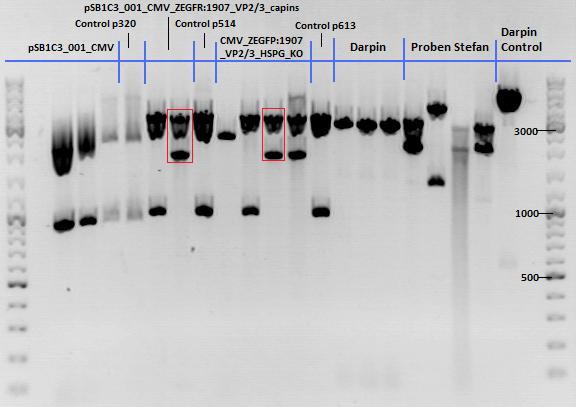
Test Digestions of yesterdays minipreps
Investigator: Achim, Anna
- 001_CMV: Cut with EcoRI, PstI & PvuII
- P654 (Lane 1)
- P655 (Lane 2)
- P686 (Lane 3)
- Control P320 (Lane 4)
- CMV_ZEGFR:1907_VP2/3_inscap: Cut with EcoRI & SspI
- P678 (Lane 5)
- P679 (Lane 6)
- Control P514 (Lane 7)
- Results:The expected bands should run at 1700 and 3000 bp. P679 was sent for sequencing.
- CMV_ZEGFR:1907_VP2/3_inscap_HSPG-KO: Cut with EcoRI & SspI
- P680 (Lane 8)
- P681 (Lane 9)
- P684 (Lane 10)
- P685 (Lane 11)
- Control P613 (Lane 12)
- Results:The expected bands should run at 1700 and 3000 bp. P684 was sent for sequencing.
- pCerulean_Vp1up_NLS_Darpin: Cut with BamHI & XbaI
- P676 (Lane 13)
- P677 (Lane 14)
- P683 (Lane 15)
- Control P365 (Lane 20)
- Results:The expected bands should run at 500 and 4500 bp. However, just one band at ~3000 bp is visible on the gel.
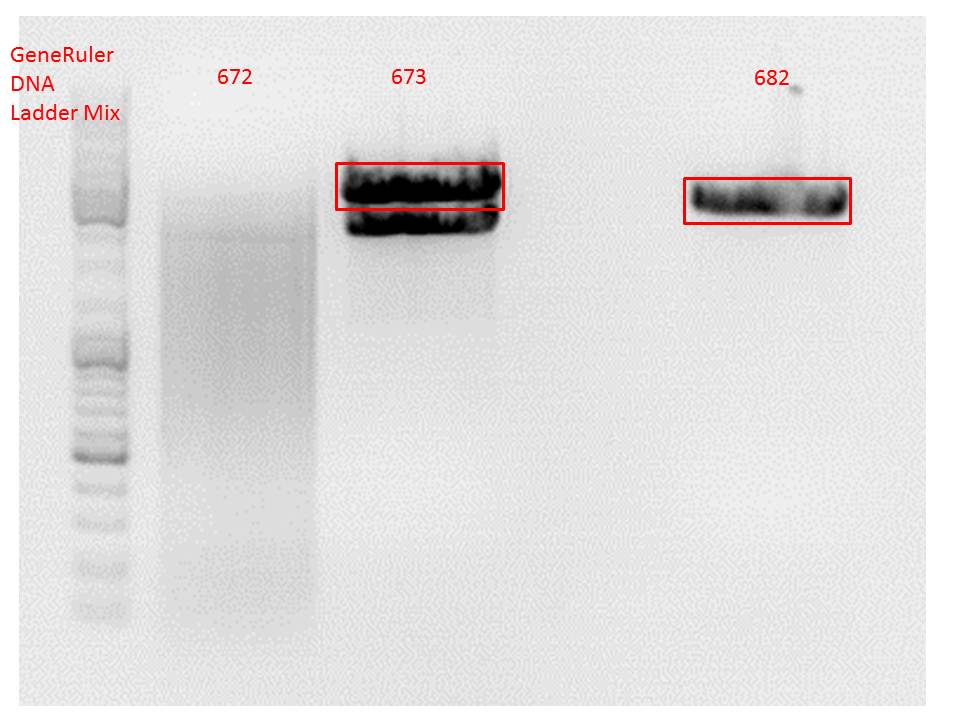
Samples Stefan: Cut with Xba & Spe
- P670 (Lane 16)
- P671 (Lane 17)
- P672 (Lane 18)
- P673 (Lane 19)

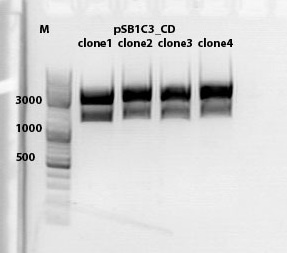
mini preps of CD clones
Investigator: Kira
c(p689)= 115 ng/ul
c(690)= 151, 20 ng/ul
c(691)= 122,27 ng/ul
c(p692) = 126,42 ng/ul
test digestion of CD clones
Investigator: Kira
| Components | sample Volume/µL |
| DNA | 4,0 µl |
| BSA (10x) | 2 µl |
| Buffer no. 4 | 2,0 µl |
| Enzyme 1 XbaI | 1,0 µl |
| Enzyme 2 AgeI | 1,5 µl |
| H2O | 9,5 µl |
| Total volume | 20 |
incubation @ 37 C for approx. 2 h
1% agarose gel
Biobrick assembly pSB1C3_lITR_hTERT_beta-globin_CD
Investigator: Kira
c(pSB1C3_lITR_hTERT_beta-globin)= 333 ng/ul
c(pSB1C3_CD)= 151 ng/ul
| Components | vector Volume/µL | insert Volume/µL |
| DNA | 4,5 µl | 6 |
| BSA (10x) | 3 µl | 3 |
| Buffer no. 4 | 3,0 µl | 3 |
| Enzyme 1 XbaI | 0 µl | 1,5 |
| Enzyme 2 SpeI | 1,5 µl | 0 |
| Enzyme 3 PstI-HF | 1,0 | 1 |
| H2O | 17 | 15,5 |
| Total volume | 25 |
incubation @ 37 C for approx. 2 h
1% agarose gel
Ligation
DNA-mix: 8 ul (vector 4,6ul)+(insert 3,4 ul)
T4 ligase: 1 ul
T4 buffer: 1 ul
Incubation @ RT for 30 min
Transformation was performed according to the standard protocol w BL21 cells.
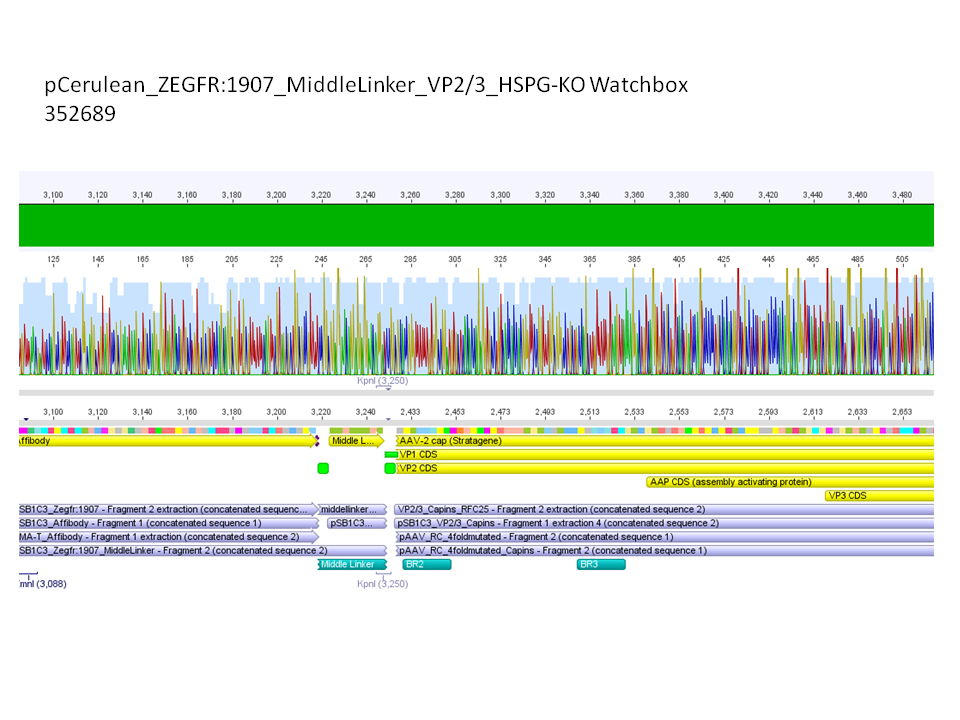
Sequencing results of pCerulean_Zegfr:1907_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Comment: All N-terminal fusion approaches with VP2/3_HSPG-KO revealed positive results except of pCerulean_Zegfr:1907_MiddleLinker_VP2/3_HSPG-KO. Another clone was picked, preped, test digested and sent for sequencing.
Conclusion: Sequencing results revealed positive results.
Picking clones of pGA14_MiddleLinker_VP2/3_insCap and pGA14_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Unfortunately there grew a bacteria lawn over night - it was hardly not possible to pick clones. Nevertheless I tried and picked 2 clones of pGA14_MiddleLinker_VP2/3_insCap and pGA14_MiddleLinker_VP2/3_HSPG-KO.
To do: Mini-Prep and test digestion.
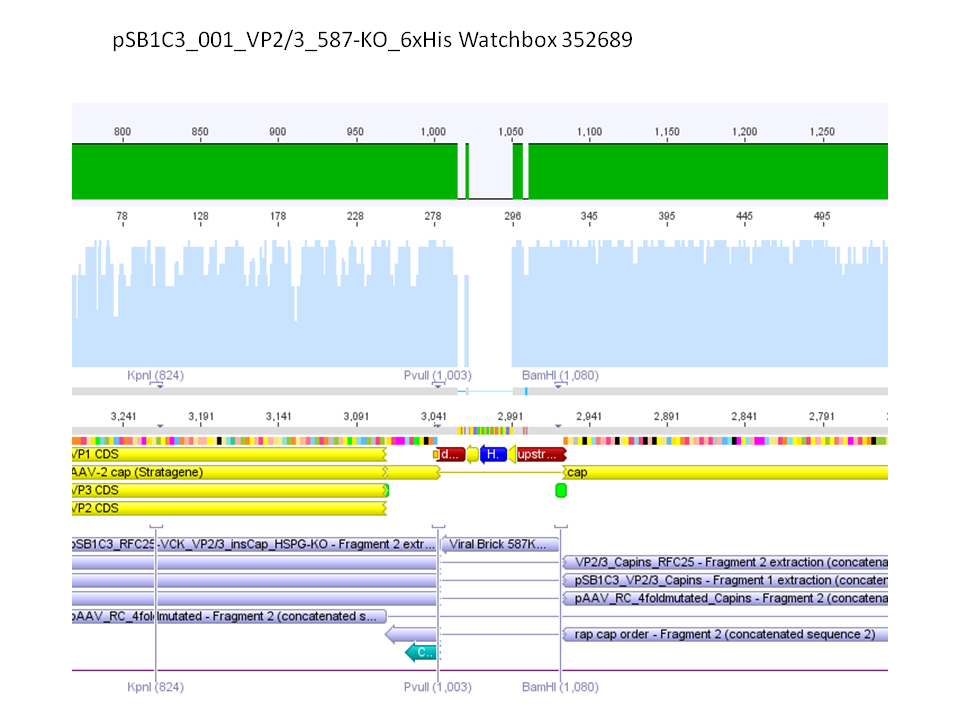
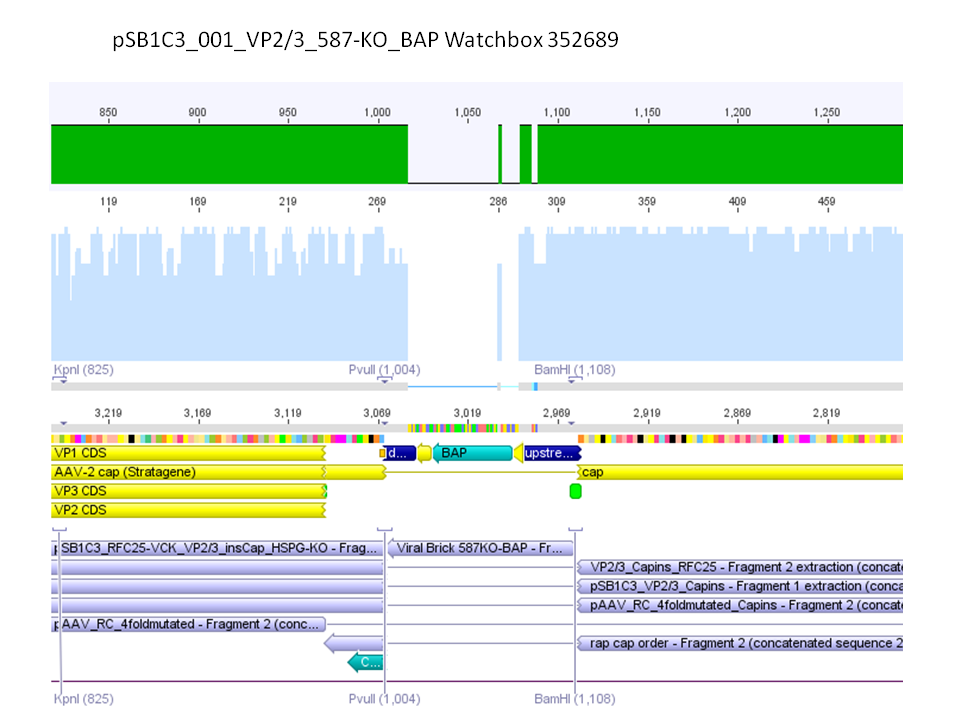
Sequencing results of pSB1C3_001_VP2/3_587-KO_BAP and pSB1C3_001_VP2/3_587-KO_6xHis
Investigator: Hanna
Comment: For the creation of our super constructs, the His-Tag and the BAP motif need to be cloned into VP2/3 for N-terminal fusion to VP2. Sequencing results showed that the 6xHis and BAP motif was not cloned into VP2/3_insCap.
To do: Clone 587-KO_6xHis and 587-KO_BAP into pSB1C3_001_VP2/3_insCap 1. via digestion of inserts and 2. via hybridization of referring oligos - digestion of vector with 800-900 ng.
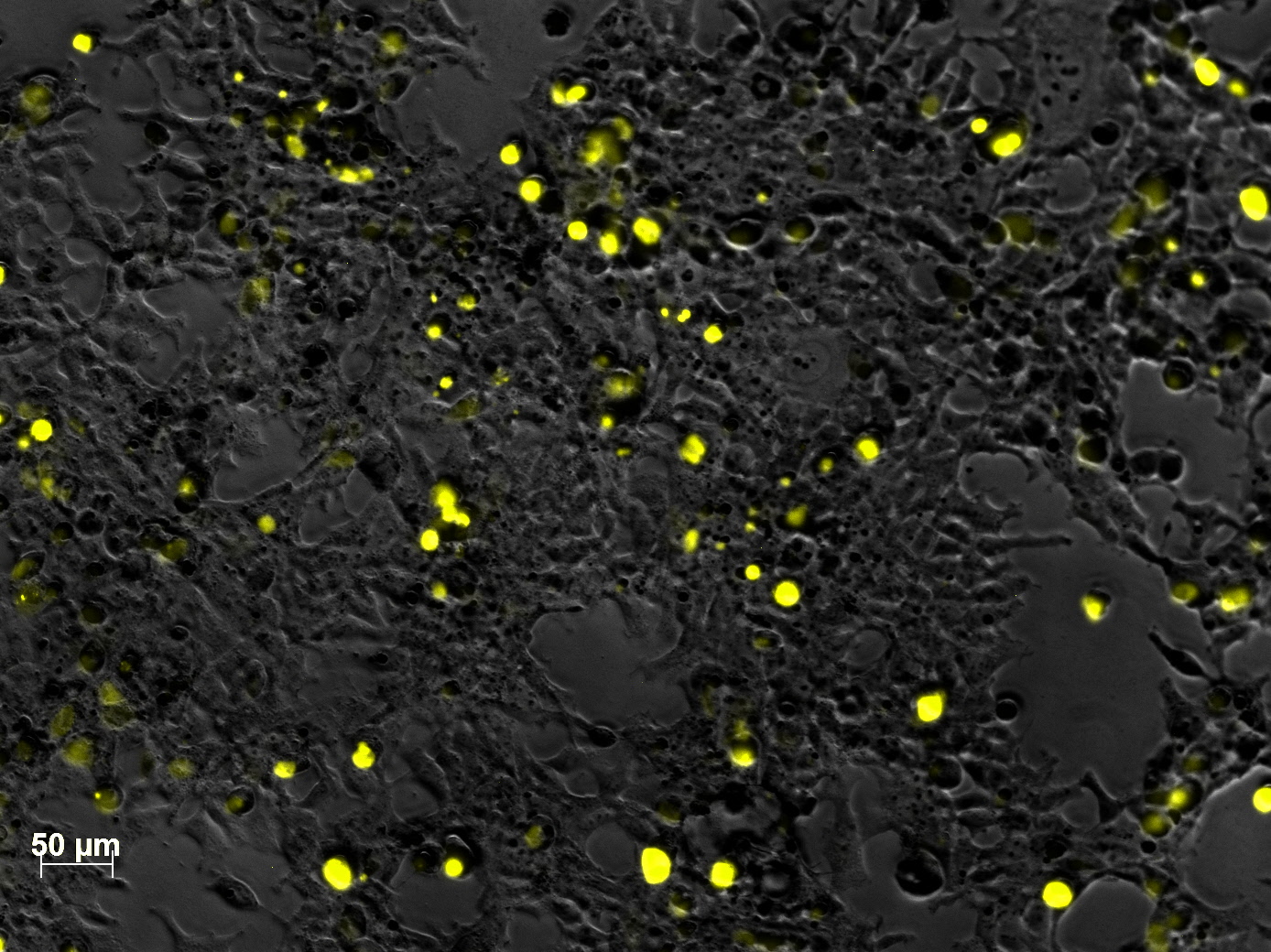
Impressions of transfection of AAV293 with pCerulean_VP1up_NLS_mVenus_VP2/3
Investigator: Adrian
Yesterday AAV293 cells were transfected with pCerulean_VP1up_NLS_mVenus_VP2/3 and GMK-TK30 as gene of interest. Today, nuclear localization of the produced mVenus_VP2/3 fusion protein was visible and can be nicely seen in the following pictures:
Conclusion: The VP2/3 fusion particle was transcribed and translated AND was transported back into the nucleus in order to be packaged by the ITR-flanked gene of interest.
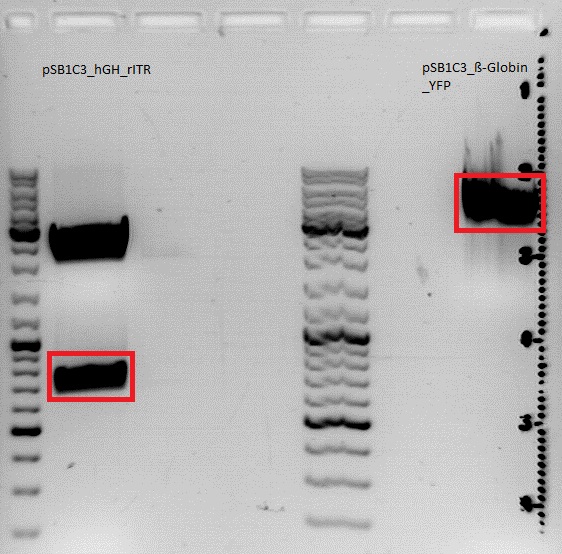
Cloning of hGH_rITR into pSB1C3_lITR_phTERT_beta-globin_CFP and lITR_phTERT_beta-globin_mGMK_TK30 into pSB1C3_hGH_rITR
Investigator: Stefan
Vector name:
- pSB1C3_lITR_phTERT_beta-globin_CFP (P682)
- pSB1C3_hGH_rITR (P186)
Insert name:
- pSB1C3_hGH_rITR (P186)
- pSB1C3_lITR_phTERT_beta-globin_mGMK_TK30 (P672)
- pSB1C3_lITR_phTERT_beta-globin_mGMK_TK30 (P673)
| components | volume for P186 /µl | volume of P186 X+E /µl | volume of P672 + P673 /µl | volume of P682 /µl |
| DNA | 12 | 12 | 5 | 14 |
| BSA (10x) | 2,5 | 2 | 2 | 2 |
| Buffer 4 (10x) | 3 | 2 | 2 | 2 |
| Enzyme XbaI | 1 | 1 | - | - |
| Enzyme PstI | 1 | - | - | 1 |
| Enzyme EcoI | - | 1 | 1 | - |
| Enzyme SpeI | - | - | 1 | 1 |
| H2O | 6 | 6 | 9 | - |
| Total volume (e.g. 15,20,25,30 µl) | 25 | 25 | 20 | 20 |
Gel:
0,5 g Agarose, 50 ml TAE (1%), 3 µl GELRED , at 115 Volt
Comment: Since the first gel revealed problems with samples 672, 673 and 682 another digestion was prepared (same approach as shown above), this time using a 0,8% gel and another loading dye (without SDS).
0,4 g Agarose, 50 ml TAE (0,8%), 3 µl GELRED , at 115 Volt
Comment: Since sample 672 showed no bands this sample was discarded and cloning continued only using 673.
Gel extraction:
Was performed according to protocol.
T4 Ligation:
Ligation was performed over night.
| ligation name | 186 (X+E) + 673 | 186 + 682 |
| volume of vector | 3,51 | 4,49 |
| volume of insert | 5,38 | 2,64 |
Transformation:
Will be done tomorrow using BL21 cells.
New BL21 cells ready to use!
Investigator: Stefan
137. labday 02.10.2010
Repetition of the approach of subcloning the ViralBricks HSPG-ko BAP and His into VP2/3 with different strategies
Investigator: Bea
Comment: Unfortunately, the last experiment revealed that subcloning of the two ViralBricks did not work out which was shown by the sequencing results. Therefore we need to repeat the approach of subcloning the ViralBricks into the pSB1C3_VP2/3 which than can be used for fusing it to the n-terminal targeting approaches. Two different strategies will be carried out which means that we are digesting the pSB1C3_Viralbricks AND hybridize the oligos (BAP and His) in order to obtain some positive results.
The vector and ViralBricks were digested with BamHi and PvuII. The vector was dephosphorylated following the standard protocol.
The vector was loaded on a 1% agarose gel before dephosphorylation was conducted. The results can be seen above in the gel picture:
Result:
The ligation was performed over night at 18°C with T4-ligase.
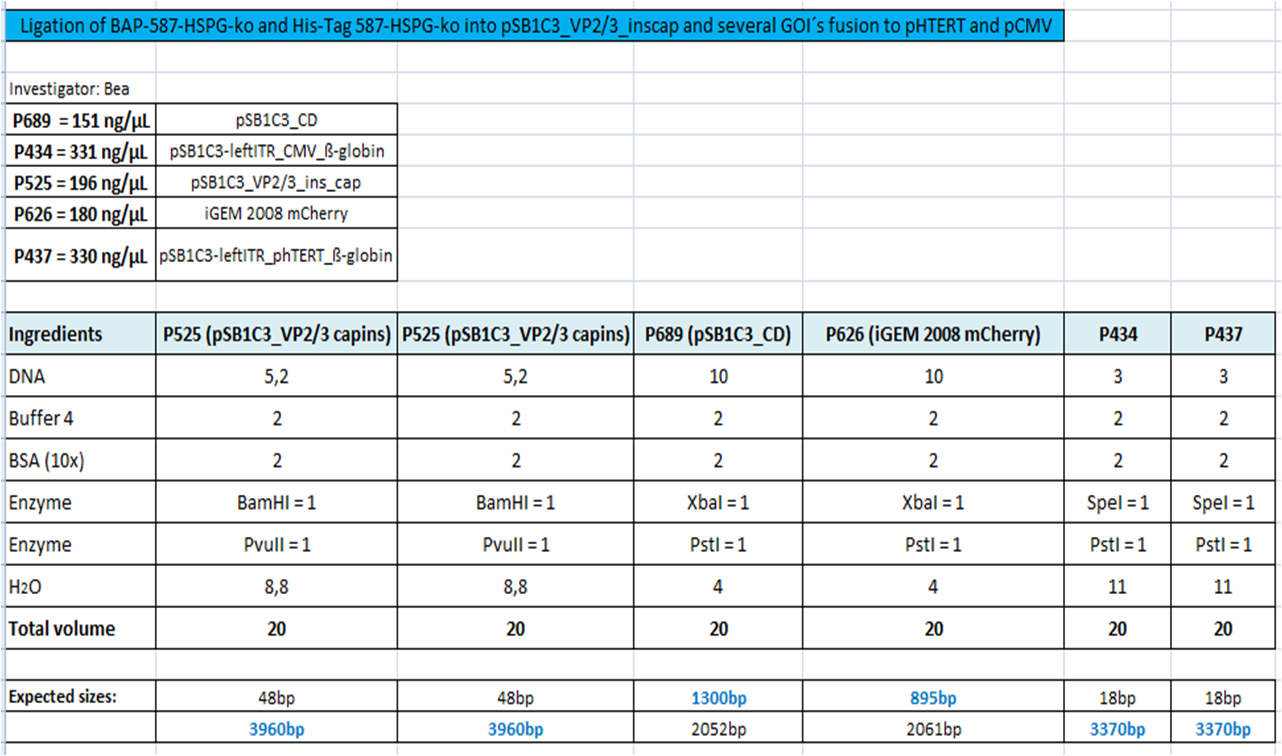
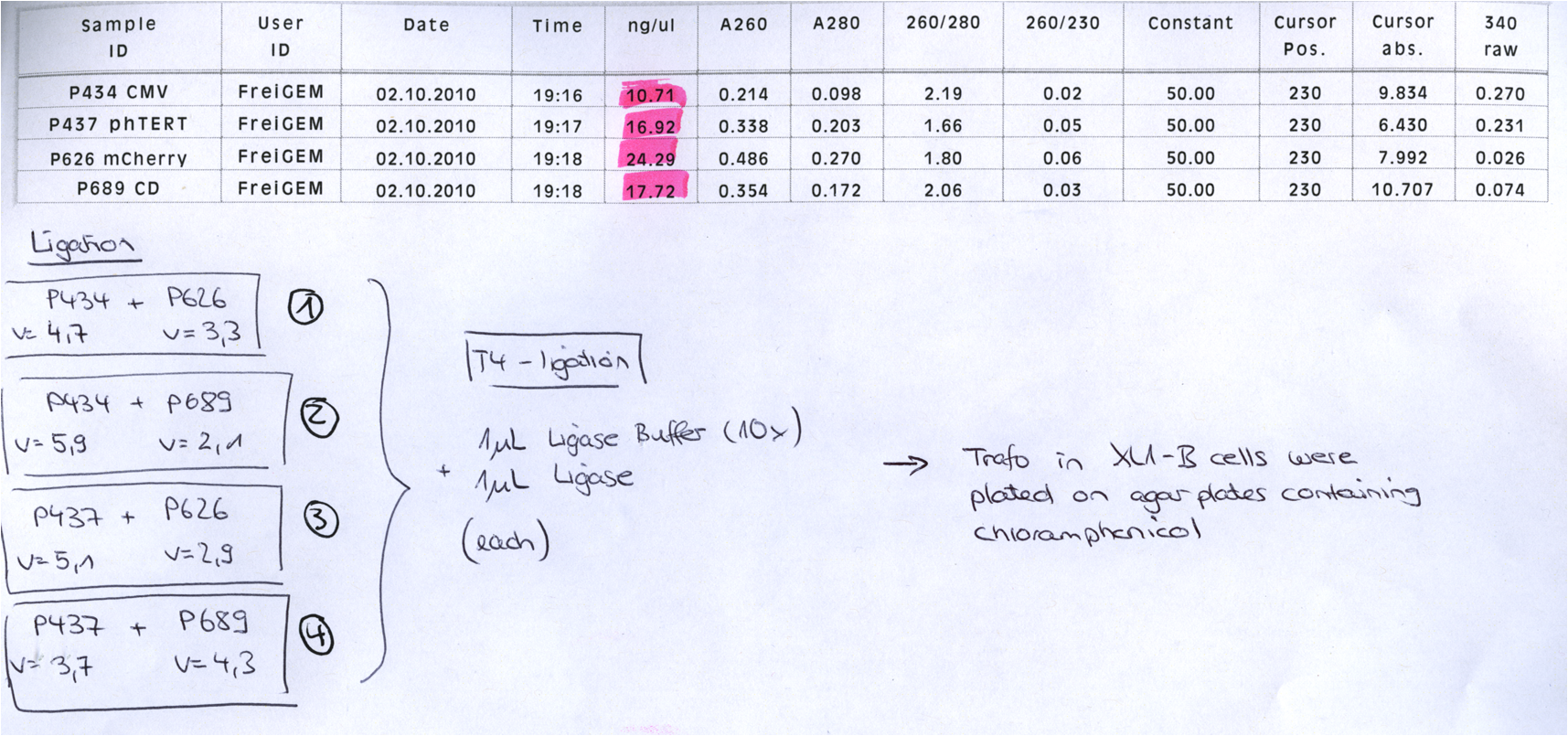
BioBrick Assembly of CD and mCherry
Investigator: Bea
Comment: We decided to choose some additional GOI´s for the vectorplasmid so the "new" GOI´s need to be fused to the leftITR_Promoter_betaglobin constructs. Since only one clone grew on the plate prepared by Kira where she fused the CD (cytosine deaminase) to the above mentioned construct I am going to repeat it aswell.
Digestion of the constructs:
- P689 = pSB1C3_CD
- P626 = iGEM_mCherry
- P434 = pSB1C3_leftITR_CMV_betaglobin
- P434 = pSB1C3_leftITR_phTERT_betaglobin
- P525 = pSB1C3_VP2/capins
Loading plan:
P689 (pSB1C3_CD) P626 (iGEM mCherry) P434 (pSB1C3_lITR_CMV_ß-globin) P437 (pSB1C3_lITR_phTERT_ß-globin)
Results:
After gel extraction has been performed, the ligation was carried out.
Ligation:
The ligation mix was transformed into XL1-Blue cells and plated on agar plates containing chloramphenicol.
Next steps:
Picking clones and perform Mini-Prep. After the Mini-Preps have been performed this construct needs to be sequenced and tested in cell culture.
comment: just one clone? uhhhh, when did you take out the plate? i put it at around 1 am..maybe they need a while to grow, no? (Kira)
Yep, I found another clone ;-) But the two look pretty good. I took them out today in the afternoon - I guess that is enough time! But if you want I can put them back into the 37° C room??! (Bea)
Mini Prep and test digestion of pGa14_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Comment: In order to fuse the DARPin to the N-terminus of VP2/3_insCap and VP2/3_HSPG-KO, these motifs need to be fused to the Middle Linker (performed on 30.9.2010). In order to be able to immediately continue with cloning, BL21 cells were transformed and clones were picked in the noon. Now, the DNA needs to be preped and test digested in order to perform a 3-fragment-ligation with the DARPin and the pSB1C3_001_CMV plasmid. Unfortunately the cells of the VP2/3_insCap construct grew not densely enough. Therefore two new clones were picked from the plate and were inoculated in 10 mL LB medium. The other construct (pGa14_MiddleLinker_VP2/3_HSPG-KO) was preped and test digested.
TEST DIGESTION:
For both clones:
- DNA: 4 µL
- Buffer 4: 1 µL
- BSA: 1 µL
- EcoRI: 0.5 µL
- PstI: 0.5 µL
- H2O: 3 µL
Incubation: 1 hour.
Agarose gel: 0.8 %, 115 Volt, 1 hour.
Comment: The choice of these restriction enzymes was not clever, because if cloning didn't work, I expect one band at 2379 bp (the insert should hardly be seen) - if it worked one band at 2379 bp and a second at 2005 bp. Fortunately the bands could be separated ans revealed positive results for both clones :

Next steps:
- Mini-Prep and test digestion of pGa14_MiddleLinker_VP2/3_ins Cap.
- 3 fragment ligation of MiddleLinker_VP2/3_ins Cap or MiddleLinker_VP2/3_HSPG-KO + DARPin + pSB1C3_001_CMV backbone.
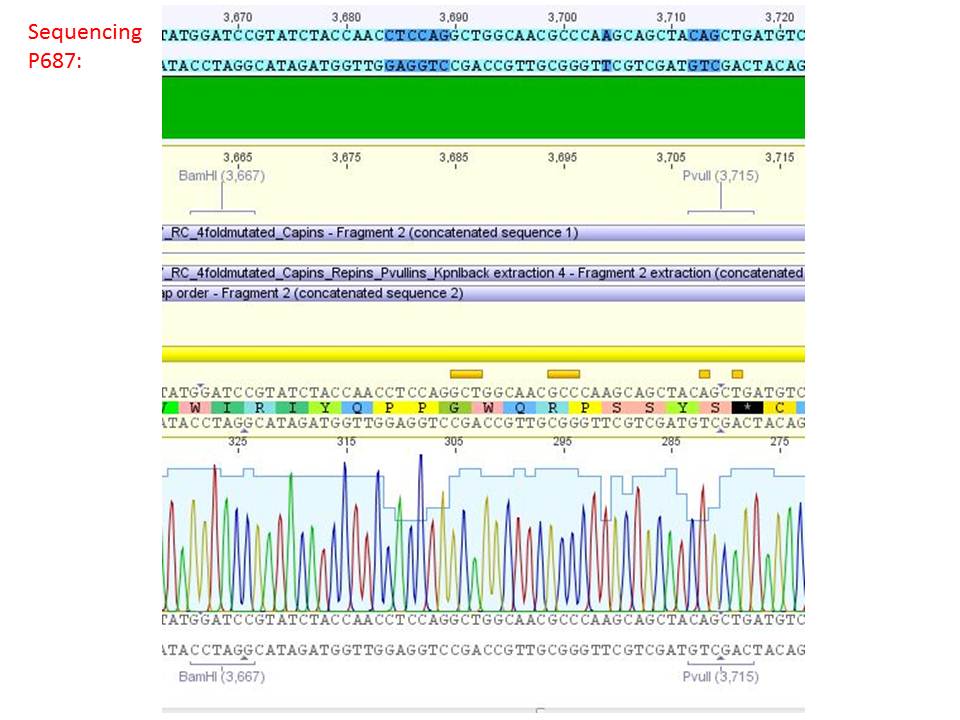
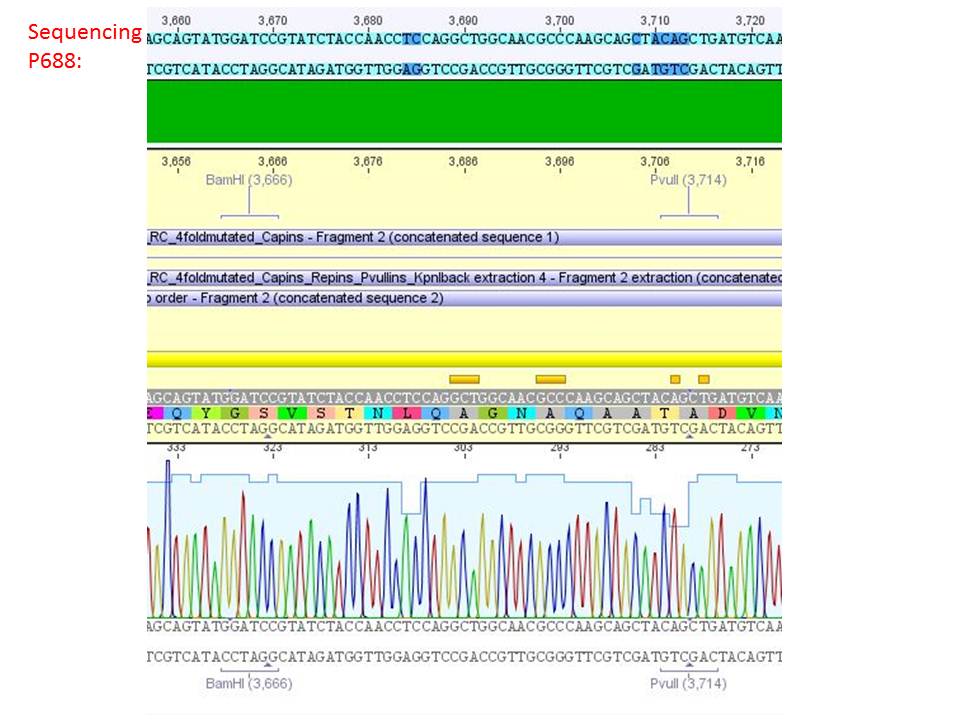
Sequencing analysis of HSPG-ko in several IRCK constructs
Investigator: Stefan
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 1 (P687):
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 2(P688):
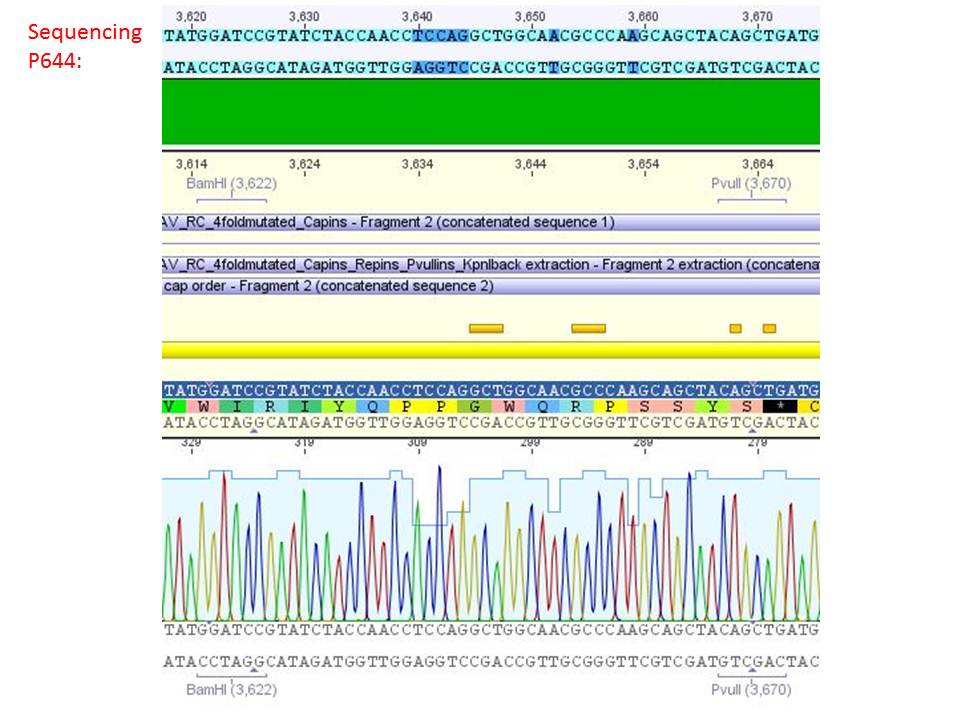
pSB1C3_001_RC_IRCK_VP1-ko_HSPG-ko_P5tataless cl1 (P644):
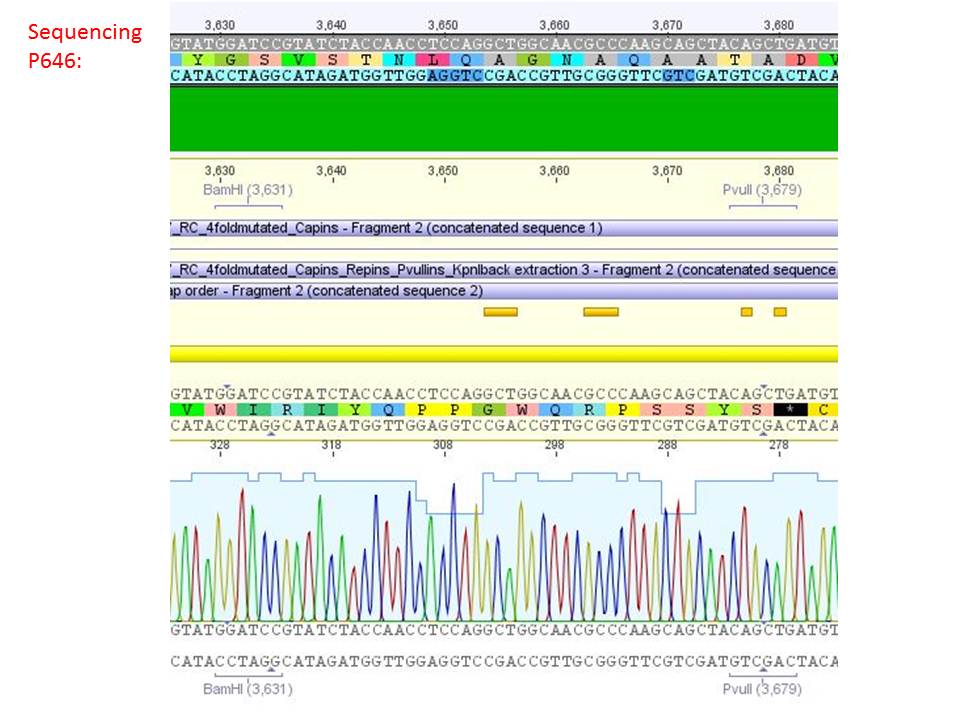
pSB1C3_001_RC_IRCK_VP2-ko_HSPG-ko_P5tataless cl1 (P646):
Comment: Sequencing results show that all plasmids contain the HSGP-ko. Working will be continued using P644, P646 and P687.
Mini Prep of pGa14_MiddleLinker_VP2/3_inscap and pSB1C3_Darpin
Investigator: Jessica
- pSB1C3_Darpin clone1 c=158.5 ng/µl
- pSB1C3_Darpin clone2 c=205.6 ng/µl
- pGA14_MiddleLinker_VP2/3_inscap clone 1 c=147.5 ng/µl
- pGA14_MiddleLinker_VP2/3_inscap clone 2 c=121.0 ng/µl
Test-digestion:
| Components | pSB1C3_Darpin clone1/2 |
| DNA | 1,5 µl |
| BSA (10x) | 1 µl |
| Buffer no. 4 | 1 µl |
| Enzyme 1 XbaI | 0,4 µl |
| Enzyme 2 AgeI | 0,4 µl |
| H2O | 5,7 µl |
| Total volume | 10 |
Hanna wrote that a 3rd enzyme is need for the test digestion of pGA14_MiddleLinker_VP2/3_inscap but I couln't find any sequence of pGA14_MiddleLinker_VP2/3_inscap or pGA14

Test digestion of pGA14_MiddleLinker_VP2/3_inscap
Investigator: Achim
- pGA14_MiddleLinker_VP2/3_inscap clone 1 c=147.5 ng/µl (P698)
- pGA14_MiddleLinker_VP2/3_inscap clone 2 c=121.0 ng/µl (P699)
- Control: pGA14_MiddleLinker (P301)
- Digestion with EcoRI & SalI; should yield two bands of 3400 & 1000 bp. The negative control should only be cut once.
Mass Midi-Prep III
Investigator: Chris W.
Midi-Prep of:
pSB1C3_001_RC_IRCK_VP1-ko_HSPG-ko_P5tataless cl1 =P700 =B521
pSB1C3_001_RC_IRCK_VP2-ko_HSPG-ko_P5tataless cl1 =P701 =B523
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 1 =P702 =B561
pCerulean_ZEGFR:1907_MiddleLinker_VP2/3_HSPG-KO clone 2 =P703 =B543
pCerulean_CFP_MiddleLinker_VP2/3_HSPG-KO =P704 =B507
pCerulean_ZEGFR:1907_SEG_VP2/3_HSPG-KO =P705 =B509
pCerulean_ZEGFR:1907_ShortLinker_VP2/3_HSPG-KO =P706 =B510
pCerulean_ZEGFR:1907_LongLinker_VP2/3_HSPG-KO =P707 =B511
pCerulean_6xHis_MiddleLinker_VP2/3_HSPG-KO =P708 =B512
pCerulean_VP1up_NLS_ZEGFR:1907_VP2/3_HSPG-KO =P709 =B513
pCerulean_VP1up_NLS_6xHis_VP2/3_HSPG-KO =P710 =B514
pCerulean_VP1up_NLS_mVenus_VP2/3_HSPG-KO =P711 =B515
pSB1C3_001_RC_IRCK_P5tataless clone 1 =P712 =B516
The Midi-Preps were performed according to the standard protocol yielding the following concentrations:
| plasmid-no. | P700 | P701 | P702 | P703 | P704 | P705 | P706 | P707 | P708 | P709 | P710 | P711 | P712 |
| concentration (ng/µl) | 695 | 370 | 601 | 1548 | 3996 | 3630 | 2460 | 2610 | 1860 | 2913 | 2964 | 3030 | 1200 |
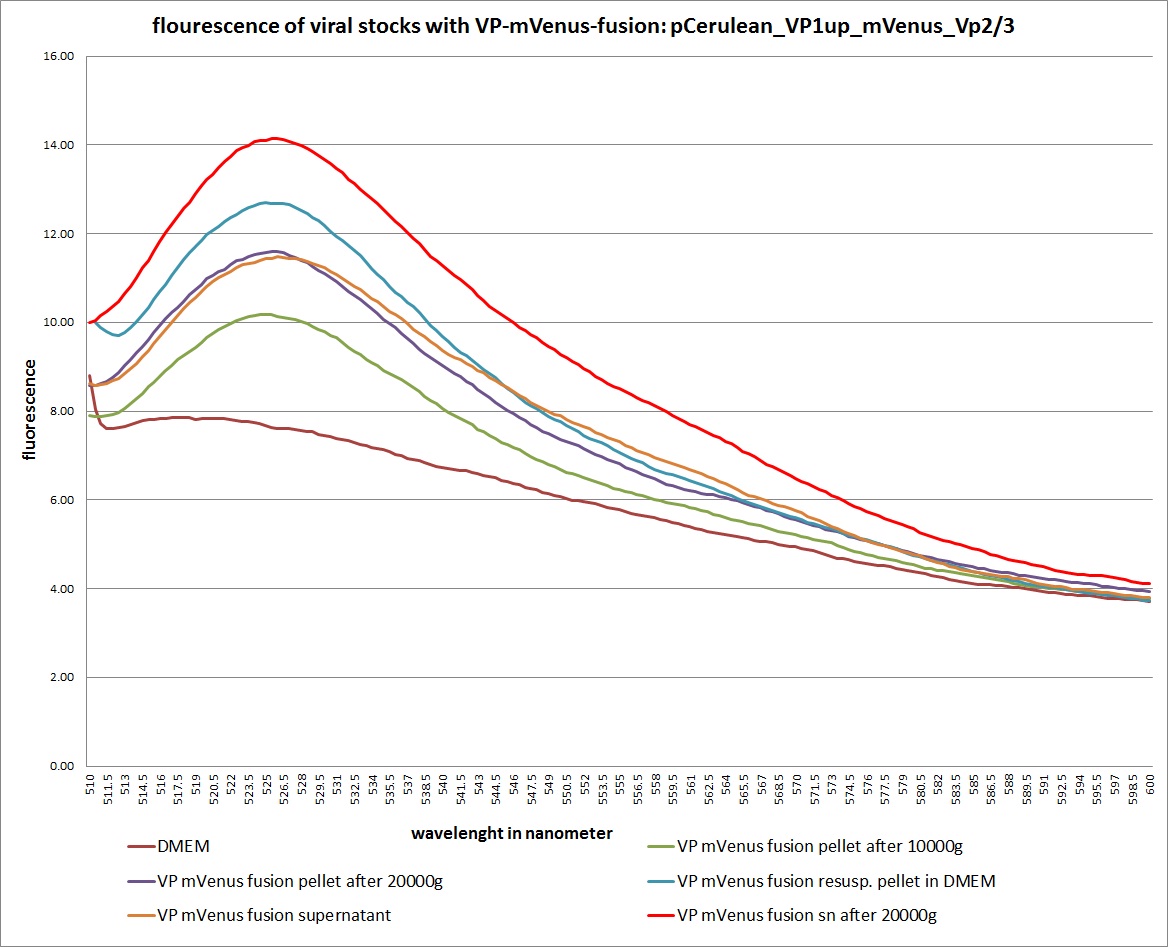
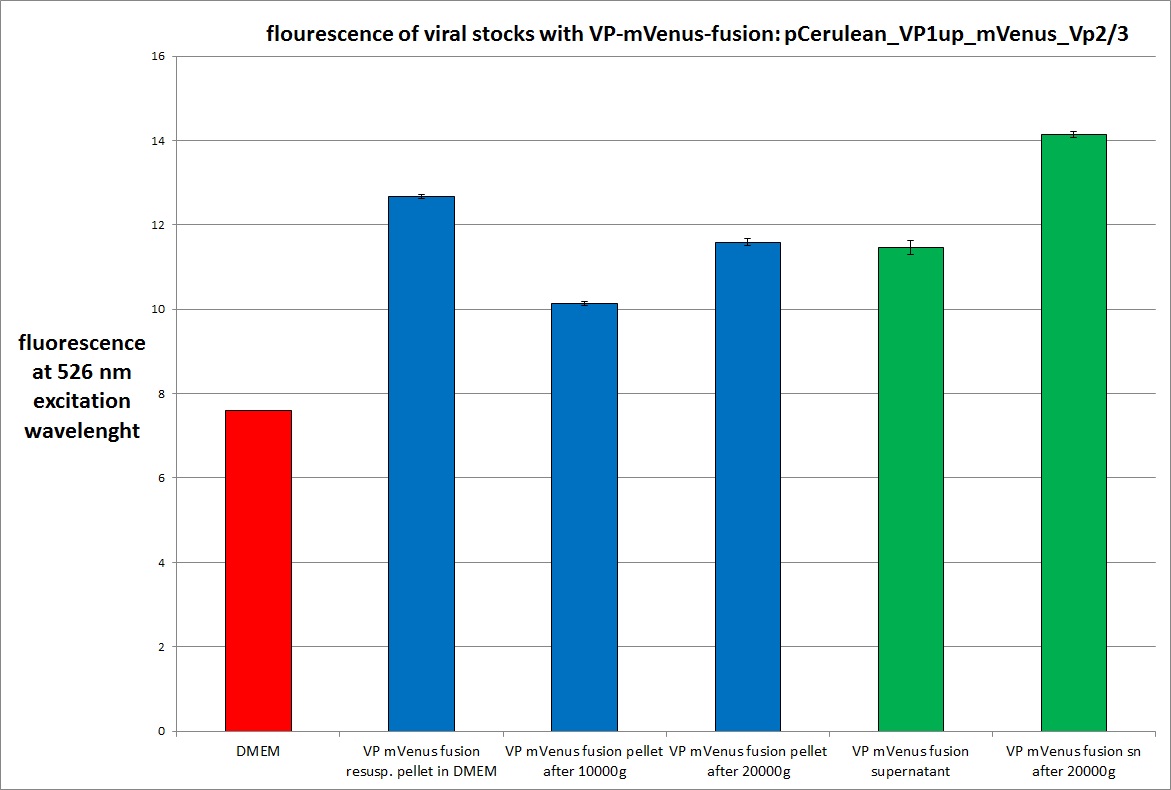
Analysis of viral harvest via spectrometer
Investigator: Adrian, Patrick
The motivation: We want to figure out if it is possible to purify our viral stocks via centrifugation with 10.000 g and/or 20.000 g. In theory/ according to the literature the viral particles should be in the cells and attached to the HSPGs at the cellular surfaces.
The plan: Two different viral stocks were prepared:
Stock 1 with VP2-mVenus-fusion-Capsid loaded with TKGMK (4 wells from a 6 well plate)
- R/C 1: 50% pCerulean_VP1up_NLS_mVenus_Vp2/3 P501
- R/C 2: 50% Rep/Cap2: P449 pAAV_RC_4fachmut_VP1-ko:
- GOI: TKGMK: P82.b
- pHelper
Stock 2 "standard" virus (4 wells from a 6 well plate)
- R/C 1 P486
- GOI: TKGMK: P82.b
- pHelper
These two stocks got harvested according to standard protocol (scratching cells, transfer them in to 15 ml falcons, resuling 12ml solution total). After centrifugation (10 min 200g), the supernatant got transferred into two new 15 ml falcons and the pellets were resuspended with 10 ml DMEM Medium. The Stocks got freezed thawed two times so finally there were:
- DMEM as negative control
- resuspended Pellet with mVenus-fusioned viral capsids
- resuspended Pellet with "standard virus"
- supernatant with mVenus-fusioned viral capsids
- supernatant with "standard virus"
The fluorescence of each stock was measured via spectrometer. After that, stocks 2 and 4 were centrifugated 10min with 10.000 g followed by an other fluorescence measuring. Finally the stocks got spin down with 20.000 g for 10 min
The results:
The conclusions:
The highest amount of fluorescence was measured in the supernatant, the individual centrifugation steps had no decrease in fluorescence as consequence!
Keep in mind, without further investigation it is not valid to say that we can purify our stocks via 10-20.000 g centrifugation steps, because we dont know if the viral capsids are still intact! The FACS analysis are not sufficient enough to make a decision, because our last samples had YFP as GOI (=> so efficient cell sorting was not possible). We need to do qPCRs to make a valid evidence.
200µl-Transduction for testing the pTERT-promoter
Investigator: Adrian, Patrick
We transduced A431, HT1080 and AAV293 cells with 4 different viral stocks:
- control vector from 15.9, 750.000 cells, 3.3 µg approach
- P25 R/C= 326 pTERT_mVenus
- P26 pAAV_RC_RepCapIns_SDMKpnI clone 1 P431 pTERT_mVenus
- His-linker-fusion
CRUCIAL: There was not enough viral stock: so the whole Transduction was performed with 200µl instead of 1000µl
There are 45 samples to FACS on monday 4.10. Tripple values for each stock of each cell lines.
Transfection
Investigators: Adrian, Patrick
Transfection followed standard protocol (3.3 µg of each plasmid, 1 ml 0.3M CaCl2 and 1 ml of 2xHBS buffer PH 11.12) but with 20 min incubation time (Ca-DNA-2xHBS)
following stocks:
- mVenus standard stock 10 x cm R/C: P4682 plates
- TKGMK stock 10 x cm2 plates R/C: P468
- viral particles with GOI (mVenus) missing HGH one 6well plate
- viral particles with GOI (mVenus) missing beta globin one 6well plate
- viral particles with R/C missing HSPG binding motif (P687) one 6well plate
- viral particles with new standard R/C (P668) one 6well plate
138. labday 03.10.2010
Proceeding with the approach of subcloning the ViralBricks HSPG-ko BAP and His into VP2/3 with different strategies
Investigator: Bea
Comment: Ligation was carried out over night and transformation will be performed today.
Since the digested vector was not sufficient for all four ligation I decided to perform the ligation only with the hybridized oligos 587-ko His and the digested Viralbricks 587-HSPG-ko His and BAP. If the digested BAP insert reverals no positive results, the hybrizied oligos for BAP can be used for another approach. The transformation was carried out in
- BL-21 cells
- and plated on agar plastes containing chloramphenicol
Midi-Prep of pSB1C3_lITR_CMV_betaglobin_mVenus_hGH_rITR clone1
Investigator: Chris W.
Midi-Preps of pSB1C3_lITR_CMV_betaglobin_mVenus_hGH_rITR clone1 =P713 =B200
The Midi-Prep were performed according to the standard protocol yielding the following concentrations:
| plasmid-no. | P713 |
| concentration (ng/µl) | 1248,3 |
139. labday 04.10.2010
Three fragment ligation: pSB1C3_001_CMV_VP1up_NLS_Targeting_HSPG-KO_VP2/3
Investigator: Achim
Four ligations in total:
- Cloning CMV & VP1up_NLS_mVenus_HSPG-KO_VP2/3 into pSB1C3_001
- Cloning CMV & VP1up_NLS_His_HSPG-KO_VP2/3 into pSB1C3_001
- Cloning CMV & VP1up_NLS_affibody_HSPG-KO_VP2/3 into pSB1C3_001
- Cloning CMV into pSB1C3_001
Used plasmids:
- pSB1C3_CMV (P145)
- c=225.5 ng/µl -> 8 µl
- Cut with EcoRI & SpeI
- pCerulean_VP1up_NLS_mVenus_HSPG-KO_VP2/3 (P711)
- c=3030 ng/µl -> 0.7 µl
- Cut with XbaI & PstI
- pCerulean_VP1up_NLS_His_HSPG-KO_VP2/3 (P710)
- c=2965 ng/µl -> 0.7 µl
- Cut with XbaI & PstI
- pCerulean_VP1up_NLS_affibody_HSPG-KO_VP2/3 (P709)
- c=2913 ng/µl -> 0.7 µl
- Cut with XbaI & PstI
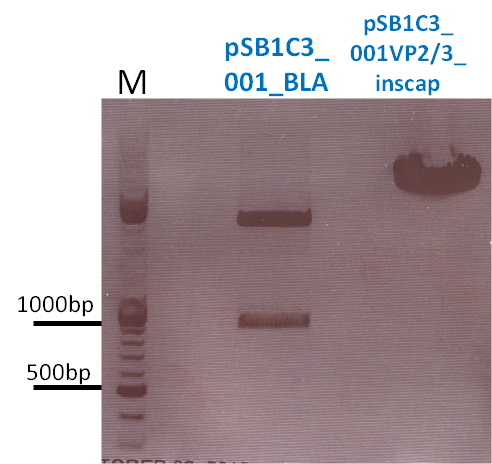
- pSB1C3_001_BLA (P320)
- c=408.5 ng/µl -> 5 µl
- Cut with EcoRI & PstI for three fragment ligations
- Cut with EcoRI & SpeI for ligation with CMV
´Seeding AAV293 for Transfection
Investigator Patrick
9 6-well plates were prepared for the transfection tomorrow. 480000 cells were seeded into each well.
FACS Analysis of pTERT_mVenus-loaded-viral particles and pCerulean_VP1up_His_VP2/3
Investigators: Kerstin, Anissa
45 samples of the three cell lines: AAV293, A431 and HT1080 got transduced (see 2.10) with following viral stocks:
1.
- pHelper
- R/C : 326
- pTERT-mVenus
2.
- pHelper
- R/C : P431
- pTERT-mVenus
3.
- pHelper
- R/C P158a
- CMV_mVenus
4.
- pHelper
- R/C: pCerulean_VP1up_His_VP2/3
- pAAV_RC_4fachmut_VP1-ko
- CMV_mVenus
Assembly of pSB1C3_CMV_DARPin_VP2/3_insCap and pSB1C3_CMV_DARPin_VP2/3_HSPG-KO
Investigators: Hanna
Comment: We also want to test the DARPin_E01 for the N-terminal fusion approaches. Today a 3 fragment ligation will be performed in order to complete the VP2 fusion constructs with the DARPin.
Because 2 test digestions showed that the fusion of the DARPin to VP1up_NLS didn't work as ecpected (insert is too large) this has to be repeated before also the VP1 insertion with the DARPin can be completed.
Digestion
| components | volume of pSB1C3_CMV /µl | volume of pG14_ML_VP2/3_insCap /µl | volume of pG14_ML_VP2/3_HSPG-KO /µl | volume of pSB1C3_001_DARPin /µl |
| DNA | 9 | 6.8 | 10 | 5 |
| BSA (10x) | 2 | 3 | 3 | 2 |
| Buffer 4 (10x) | 2 | 3 | 3 | 2 |
| SpeI | 1 | - | - | - |
| PstI | 1 | 1 | 1 | - |
| NgoMIV | - | 1 | 1 | - |
| XmnI | - | 1 | 1 | - |
| XbaI | - | - | - | 4 |
| H2O | 5 | 14.2 | 11 | 9 |
| Total volume | 20 | 30 | 30 | 20 |
- Incubation: 2.5 h
Agarose-Gel:
0.5 g Agarose, 50 mL TAE (1 %), 3 µL GELRED, at 115 Volt, running time: 45 minutes
| Sample | Sample/µl] | Loading dye (6x)/µl | Expected size 1 |
|---|---|---|---|
| CMV | 20 µl | 4 µl | ~ 2700 bp |
| MiddleLinker_VP2/3_insCap | 30 µl | 4 µl | ~ 2000 bp |
| MiddleLinker_VP2/3_HSPG-KO | 30 µl | 4 µl | ~ 2000 bp |
| DARPin | 20 µl | 4 µl | ~ 475 bp |
Gelex
- CMV: 48.99 ng/µL
- MiddleLinker_VP2/3_insCap: 12.95 ng/µL
- MiddleLinker_VP2/3_HSPG-KO ng/µL
- DARPin: 9.59 ng/µL
Ligation
| Construct | CMV (µl) | MiddleLinker_VP2/3_insCap (µl) | DARPin (µl) |
| pSB1C3_CMV_DARPin_MiddleLinker_VP2/3_insCap | 1 | 3.5 | 3.5 |
| Construct | CMV (µl) | MiddleLinker_VP2/3_HSPG-KO (µl) | DARPin (µl) |
| pSB1C3_CMV_DARPin_MiddleLinker_VP2/3_HSPG-KO | 1 | 3.5 | 3.5 |
Trafo
Was performed following the standard protocol.
Cells: XL1b
Miniprep of serveral constructs
Investigator: Stefan
Glycerol stocks were prepared:
B573 pSB1C3_lITR_CMV_betaglobin_mCherry clone1
B574 pSB1C3_lITR_CMV_betaglobin_mCherry clone2
B575 pSB1C3_lITR_CMV_betaglobin_CD clone1
B576 pSB1C3_lITR_CMV_betaglobin_CD clone2
B577 pSB1C3_lITR_phTERT_betaglobin_mCherry clone1
B578 pSB1C3_lITR_phTERT_betaglobin_mCherry clone2
B579 pSB1C3_lITR_phTERT_betaglobin_CFP_hgH_rITR clone 1
B580 pSB1C3_lITR_phTERT_betaglobin_CFP_hgH_rITR clone 2
B581 pSB1C3_lITR_phTERT_betaglobin_CFP_hgH_rITR clone 1
B582 pSB1C3_lITR_phTERT_betaglobin_CFP_hgH_rITR clone 2
B583 pSB1C3_lITR_phTERT_betaglobin_CD clone 1
B584 pSB1C3_lITR_phTERT_betaglobin_CD clone 2
B585 pSB1C3_lITR_phTERT_betaglobin_CD clone 3
B586 pSB1C3_001_VP2/3_capins_587_KO_His_clone1
B587 pSB1C3_001_VP2/3_capins_587_KO_His_clone2
B588 pSB1C3_001_VP2/3_capins_587_KO_His_clone3
B589 pSB1C3_001_VP2/3_capins_587_KO_His_oligo_clone1
B590 pSB1C3_001_VP2/3_capins_587_KO_His_oligo_clone2
B591 pSB1C3_001_VP2/3_capins_587_KO_His_oligo_clone3
B592 pSB1C3_001_VP2/3_capins_587_KO_Bap_clone1
B593 pSB1C3_001_VP2/3_capins_587_KO_Bap_clone2
B594 pSB1C3_001_VP2/3_capins_587_KO_Bap_clone3
Mini-Prep was performed according to the standard protocol
P714 pSB1C3_lITR_CMV_betaglobin_mCherry clone1 c = 89,3 ng/µl
P715 pSB1C3_lITR_CMV_betaglobin_mCherry clone2 c = 312,4 ng/µl
P716 pSB1C3_lITR_CMV_betaglobin_CD clone1 c = 254,4 ng/µl
P717 pSB1C3_lITR_CMV_betaglobin_CD clone2 c = 314,8 ng/µl
P718 pSB1C3_lITR_phTERT_betaglobin_mCherry clone1 c = 409,8 ng/µl
P719 pSB1C3_lITR_phTERT_betaglobin_mCherry clone2 c = 322,1 ng/µl
P720 pSB1C3_lITR_phTERT_betaglobin_CFP_hgH_rITR clone 1 c = 265,1 ng/µl
P721 pSB1C3_lITR_phTERT_betaglobin_CFP_hgH_rITR clone 2 c = 238,8 ng/µl
P722 pSB1C3_lITR_phTERT_betaglobin_mGMK_TK30_hgH_rITR clone 1 c = 145,9 ng/µl
P723 pSB1C3_lITR_phTERT_betaglobin_mGMK_TK30_hgH_rITR clone 2 c = 100,5 ng/µl
P724 pSB1C3_lITR_phTERT_betaglobin_CD clone 1 c = 176,9 ng/µl
P725 pSB1C3_lITR_phTERT_betaglobin_CD clone 2 c = 165,0 ng/µl
P726 pSB1C3_lITR_phTERT_betaglobin_CD clone 3 c = 149,0 ng/µl
P727 pSB1C3_CMV c = 225,5 ng/µl
P728 pSB1C3_hGH_rITR c = 129,6 ng/µl
P729 pSB1C3_leftITR_CMV_beta-globin c = 243,4 ng/µl
P730 pSB1C3_leftITR_hTERT_beta-globin c = 81,8 ng/µl
P731 pSB1C3_001_VP2/3_insCap c = 466,1 ng/µl
P732 pSB1C3_001_VP2/3_capins_587_KO_His_clone1 c = 107,6 ng/µl
P733 pSB1C3_001_VP2/3_capins_587_KO_His_clone2 c = 110,3 ng/µl
P734 pSB1C3_001_VP2/3_capins_587_KO_His_clone3 c = 121,7 ng/µl
P735 pSB1C3_001_VP2/3_capins_587_KO_His_oligo_clone1 c = 101,0 ng/µl
P736 pSB1C3_001_VP2/3_capins_587_KO_His_oligo_clone2 c = 101,6 ng/µl
P737 pSB1C3_001_VP2/3_capins_587_KO_His_oligo_clone3 c = 87,0 ng/µl
P738 pSB1C3_001_VP2/3_capins_587_KO_Bap_clone1 c = 106,5 ng/µl
P739 pSB1C3_001_VP2/3_capins_587_KO_Bap_clone2 c = 110,2 ng/µl
P740 pSB1C3_001_VP2/3_capins_587_KO_Bap_clone3 c = 71,46 ng/µl
P741 pGGTBT7-ZEGFR:1907_Middlelinker_EGFP_His c = 115,4 ng/µl
140. labday 05.10.2010
Site-directed mutagenesis in pSB1C3_mGMK_TK30 construct
Investigator: Bea
Comment: Since we are still waiting for the ordered TK30, idea was to mutate the PstI site in the TK30 region. After mutating the PstI it is ready for submitting. Addtionally the ordered and received sr3ß can be subcloned.
Cellculture: Bad news
Something happened to our virus-production cell line AAV293.
Nearly all AAV293 cells are dead.
The whole Loop-Insertion Transfection from 3.10 is lost, our actual culture flasks aswell. We dont know the cause of this cellular mass mortality.
Repetition of pCerulean_VP1up_NLS_DARPin
Investigators: Achim
- The Test Digestion (1.10.) of the last attempts clones didn't look convincing. We sent clone 1 for sequencing yesterday, but we didn't recieve any results yet. That's why I decided to repeat the experiment today, in case results are negative.
- Details: See first run from 28.9.
Prep. Gel:
Harvest of viral stocks
Investigators:
Number of viral stocks: 4
- mVenus standard stock 10 x cm R/C: P4682 plates => Harvest in 2 50 ml Falcons, four times freeze thaw centrifugation step with 2500g for 10 min
- TKGMK stock 10 x cm2 plates R/C: P468 => Harvest in 2 50 ml Falcons, four times freeze thaw centrifugation step with 2500g for 10 min
- viral particles with GOI (mVenus) missing HGH one 6well plate => Harvest in 1 50 ml Falcon, four times freeze thaw centrifugation step with 2500g for 10 min
- viral particles with GOI (mVenus) missing beta globin one 6well plate => Harvest in 1 50 ml Falcon, four times freeze thaw centrifugation step with 2500g for 10 min
- viral particles with R/C missing HSPG binding motif one 6well plate => Harvest in 1 50 ml Falcon, four times freeze thaw centrifugation step with 2500g for 10 min
- viral particles with new standard R/C (intact HSPG binding motif) one 6well plate => Harvest in 1 50 ml Falcon, four times freeze thaw centrifugation step with 2500g for 10 min
Motivation:
The two new standard vectors are from now on called Control Vectors, to get valide data. The TKGMK Stock will be used for the MTT-ASSAY.
Repetition of Cloning lITR_phTERT_beta-globin_mGMK_TK30 into pSB1C3_hGH_rITR
Investigator: Anna
Comment: Second approach of Cloning lITR_phTERT_beta-globin_mGMK_TK30 into pSB1C3_hGH_rITR, first one didn't work (see labday ).
</p>
Vector name:
- pSB1C3_hGH_rITR (P186)
c= 129,6 ng/µl
Insert name:
- pSB1C3_lITR_phTERT_beta-globin_mGMK_TK30 (P673)
c= 266,4 ng/µl
| components | volume for Pp728 /µl | volume of P673 /µl |
| DNA | 11,6 | 7,5 |
| BSA (10x) | 2 | 2 |
| Buffer 4 (10x) | 2 | 2 |
| Enzyme XbaI | 1 | - |
| Enzyme EcoRI | 1 | 1 |
| Enzyme SpeI | - | 1 |
| H2O | 2,4 | 6,5 |
| Total volume (e.g. 15,20,25,30 µl) | 20 | 20 |
0,4 g Agarose, 50 ml TAE (0,8%), 3 µl GELRED , at 115 Volt
Gel extraction:
Was performed according to the standard protocol.
| rITR_hgH | lITR_phTERT_betaglobin_mGMK_TK30 | |
| Expected size of fragment | 2690 bp | 3045 bp |
| DNA-concentration [ng/µl] | 37,4 | 19,81 |
T4 Ligation:
| volume of vector | 1,1 µl |
| volume of insert | 6,9 µl |
Transformation:
Will be done tomorrow using BL21 cells.
Seeding HT1080 and AAV431 for transduction
- 3*6well plates HT1080
- 3*6well plates A431
for transduction with following viral stocks:
- mVenus Control Vector to quantify the other stocks
- mVenus without beta-globin
- mVenus without HGH
- mVenus in viral vectors without HSPG-Binding-site(P667)
- mVenus in viral vectors with HSPG-Binding-site, the new state-of-the-art-R/C (P668)
Cloning of pSB1C3_lITR_phTERT_beta-globin_CD and pSB1C3_lITR_CMV_beta-globin_CD
Investigator: Anissa
Vector name:
- pSB1C3_lITR_phTERT_beta-globin (p730)
- pSB1C3_lITR_CMV_beta-globin (P729)
- VB:pSB1C3_CD(P690)
Insert name:
| components | volume of P730 /µl | volume of P729 /µl | volume of P690 /µl |
| DNA | 12 | 4,1 | 9,9 |
| BSA (10x) | 2 | 1,5 | 2 |
| Buffer 4 (10x) | 2 | 1,5 | 2 |
| Enzyme AgeI | 1 | 1 | - |
| Enzyme NgoMIV | - | - | 1 |
| Enzyme PstI | 1 | 1 | 1 |
| H2O | 1 | 5,9 | 4,1 |
| Total volume (e.g. 15,20,25,30 µl) | 20 | 15 | 20 |
Gel:
for vectors and insert :
0,5 g Agarose, 50 ml TAE (1%), 3 µl GELRED , at 110 Volt
Gel extraction:
Was performed according to protocol.
T4 Ligation:
- Volume vector P729: 1,39 µl
- Volume insert: 6,61 µl
- Volume vector P730: 3,55 µl
- Volume insert: 4,45 µl
Transformation:
Was performed according to standard protocol using BL21 cells.
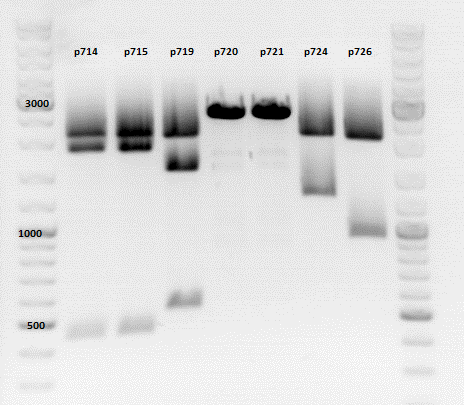
Repetition of the test-digestion of p714,p715,p719,p720,p721,p724 and p726
Investigator: Anissa
- pSB1C3_lITR_CMV_beta-globin_mCherry: Cut with EcoRI, PstI & NdeI
- P714 (Lane 1)
- P715 (Lane 2)
- Results:Looks good.
- pSB1C3_lITR_phTERT_beta-globin_mCherry: Cut with EcoRI, PstI & SacII
- P719 (Lane 3)
- Results:Looks good.
- P719 (Lane 3)
- pSB1C3_lITR_phTERT_beta-globin_CFP_hgh_rITR: Cut with EcoRI, AgeI & PstII
- P720 (Lane 4)
- P721 (Lane 5)
- Results:The expected bands should run at 671 bp,2033 bp and 1855 bp. Sequencing has to be repeated
- pSB1C3_lITR_phTERT_beta-globin_CD: Cut with PstI & EcoRI
- P724 (Lane 6)
- P726 (Lane 7)
- Results:The expected bands should run at 2035 and 2460 bp. The experiment has been repeated already today.
Repetition: Cloning of VP2/3_BAP_HSPG-KO and VP2/3_His_HSPG-KO into pCerulean_Zegfr:1907_Middlelinker and VP2/3_His_HSPG-KO into pCerulean_VP1up_NLS_mVenus
Investigator: Stefan
Comment: Cloning of 587-ko_His and 587-ko_BAP into pSB1C3_001_VP2/3_capins did not work out last time. Since cloning of VP2/3_capins started before sequencing results arrived, this experiment has to be repeated.
Vector name:
- pCerulean_Zegfr:1907_Middlelinker (P408)
- pCerulean_VP1up_NLS_mVenus (P426)
Insert name:
- pSB1C3_001_VP2/3_capins_587_KO_His_clone3 (P734)
- pSB1C3_001_VP2/3_capins_587_KO_Bap_clone1 (P738)
| components | volume for inserts (P734 + P738) /µl | volume of P408 /µl | volume of P426 /µl |
| DNA | 14 | 3 | 3 |
| BSA (10x) | 3 | 2 | 2 |
| Buffer 4 (10x) | 3 | 2 | 2 |
| Enzyme NgoMIV | 1 | - | - |
| Enzyme PstI | 1 | 1 | 1 |
| Enzyme MscI | 1 | - | - |
| Enzyme AgeI | - | 1 | 1 |
| H2O | 7 | 11 | 11 |
| Total volume (e.g. 15,20,25,30 µl) | 30 | 20 | 20 |
Gel:
0,4 g Agarose, 50 ml TAE (0,8%), 3 µl GELRED , at 115 Volt
Gel extraction:
Was performed according to protocol.
T4 Ligation:
| ligation name | 408 + 734 | 408 + 738 | 426 + 734 | 426 + 738 |
| volume of vector | 3,81 | 3,81 | 3,56 | 3,56 |
| volume of insert | 4,19 | 4,19 | 4,44 | 4,44 |
Transformation:
Was performed according to standard protocol using BL21 cells.
141. labday 06.10.2010
142. labday 07.10.2010
143. labday 08.10.2010
144. labday 09.10.2010
145. labday 10.10.2010
 "
"