|
SEPTEMBER: WEEK 5
September, 27th
MiniPrep and E-P digest for I62-a, I62-b, I62-c, I62-d, I62-e, I62-f
DNA pcr for MyYeast 1-2-3
Gel run
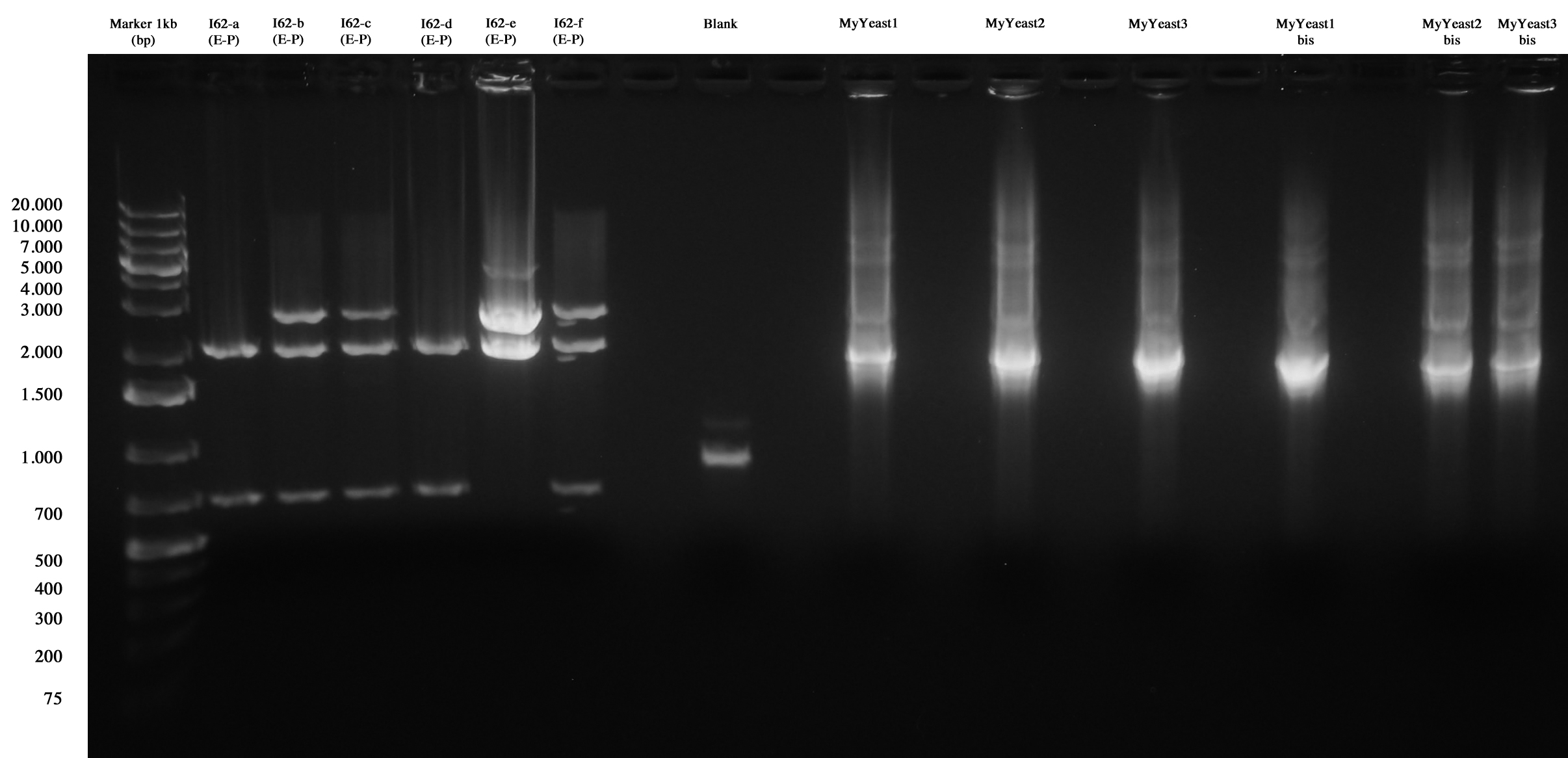 I62 and MyYeast screening.
I62-e was positive, so we made glycerol stock and stored it at -80°C.
Protein electrophoresis for phasins (Fede)
September, 28th
Tecan Test Giacomo
Miniprep and quantification for:
- I1: 59,6 ng/ul
- I4: 109,9 ng/ul
- I53: 160,3 ng/ul
- <partinfo>BBa_J04450</partinfo>-1:55,9 ng/ul
- <partinfo>BBa_J04450</partinfo>-2: 47 ng/ul
ON digestion of:
- I1 (E-S)
- I4 (E-S)
- I53 (E-X)
- <partinfo>BBa_J04450</partinfo>-1 (E-P)
- <partinfo>BBa_J04450</partinfo>-2 (E-P)
- MyYeast-3 (DNA already available) (E-S)
- <partinfo>pSB4C5</partinfo> (DNA already available) (E-P)
September, 29th
Tecan Test Giacomo
Colony PCR for 6 newly picked colonies.
 I55 colony PCR screening. No positives nor this time.
Gel run for all digested parts
and cut/gel purification for:
- 4C5 (E-P): 19,3 ng/ul
- My Yeast 3 (E-S): 20,8 ng/ul
- I1: 3,8 ng/ul
- I4 (E-S): 14,2 ng/ul
- I53 (E-X): 21,6 ng/ul
- <partinfo>BBa_J04450</partinfo> (pLac-RBS-mRFP-TT) (E-P): 5,6 ng/ul
- <partinfo>pSB1C3</partinfo>: 10 ng/ul
Ligation of:
| I70=MyYeast 3(E-S)+I4(E-S)
|
| I71=MyYeast 3(E-S)+I1(E-S)
|
| I72=<partinfo>BBa_J04450</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>
|
| I73= I38 (E-S) + I53 (E-X)
|
September, 30th
Tecan Test Nico
Transformation of I73 (1ul) into E. coli TOP10. It was plated on LB+Amp 100 ug/ml agar plate.
Trasformation of:
- I70 (myYeast with I4, Ptef1-mOrange-tAdh1) in TOP10 and plated on LB+Amp
- I71 (myYeast with I1, mOrange-tAdh1) in TOP10 and plated on LB+Amp
- I72 (pLac-RFP in pSB4C5) in TOP10 and plated on LB+Cm 12,5
All plates were incubated at 37°C ON
Since I55 was negative again we decided to ligate it again (probably we had a bad digest for vector - I20 - and a good one for insert - we used I38 in other ligations without problems). We diegested already miniprepped DNA for three hours with 1 ul of enzymes:
Gel run/cut/extraction/quantification
I20 E-X: 13,4 ng/ul
We repeated I55 ligation using newly digested vector and already digested I38 (E-S)
I55: I38 (E-S) + I20 (E-X)
Inoculum into 5ml LB+Amp of:
- I31
- I35
- I37
- I57
- I60
- INTEIN
- 3x <partinfo>BBa_J04450</partinfo> (to take <partinfo>pSB1C3</partinfo>, so <partinfo>pSB1C3</partinfo> from now on)
Cultures were let grow ON at 37°C, 220 rpm.
Inoculum of E. coli TOP10 in 1,5 ml LB, grown ON at 37°C 220 rpm. Tomorrow we will prepare competent cells!!
Inoculum of:
- S1 in 500ul M9+Cm 12,5
- I47-S1 in 500ul M9+Amp+Cm 12,5
- GFP-S1 in 500ul M9+Amp+Cm 12,5
- RBS32 in 500ul M9+Amp
Cultures were grown ON at 37°C 220 rpm.
Tomorrow we will test the ability of these str.ains to produce GFP and to lyse when HSL is added.
October, 1st
This morning, we checked tranformed plates:
- I72 showed NO colony!! :/ we will repeat this ligation next week
- I70 and I71 showed colonies, 5 for each plate were picked and inoculated in 1ml LB+Amp to prepare glycerol stocks and for screening.
- I73 showed colonies; six of them were picked and inoculated into 5 ml LB+Amp and let grow ON at 37°C, 220rpm in order to do E-P screeening the following day.
Transformation of I55 into E. coli DH5-alpha. It was plated on LB+Amp 100 agar plate and let grow ON at +37°C.
Miniprep and Nanodrop quantification for:
- I31: 76,1 ng/ul
- I35: 66,8 ng/ul
- I60: 279,7 ng/ul
(they were also sent sequencing)
- INTEIN: 123,8 ng/ul
- <partinfo>pSB1C3</partinfo>-1: 104,8 ng/ul
- <partinfo>pSB1C3</partinfo>-2: 141,2 ng/ul
- <partinfo>pSB1C3</partinfo>-3: 113,5 ng/ul
- I37: 174,9 ng/ul
Digest:
- <partinfo>pSB1C3</partinfo>: E-P
- <partinfo>pSB1C3</partinfo>: E-S
- <partinfo>pSB1C3</partinfo>: X-P
- INTEIN: E-P
- I60: E-S
- I31: S-P
- I35: E-P
- I35: E-X
- I37: E-X
- I57: E-X
Gel run/cut/extraction/quantification:
 intein, I31, I35 EX EP I37 I57 I60 |  <partinfo>pSB1C3</partinfo> digested E-P (both vector and insert were kept), E-S, X-P. |
- I37 (E-X): 20,4 ng/ul
- I57 (E-X): 17,6 ng/ul
- I60 (E-S): 9,5 ng/ul
They were stored at -20°C.
- I31 (E-S): 5,4 ng/ul
- I35 (E-P): 1,0 ng/ul ;-(
- I35 (E-X): 14,4 ng/ul
- <partinfo>pSB1C3</partinfo> (E-P): 14,3 ng/ul
- <partinfo>pSB1C3</partinfo> (E-S): 15,2 ng/ul
- <partinfo>pSB1C3</partinfo> (X-P): 13,9 ng/ul
ON ligation of:
- I74: I31 (E-S) + I35 (E-X)
- INTEIN_C3: INTEIN (E-P) + <partinfo>pSB1C3</partinfo> (E-P)
October, 2nd
PCR from colony for I55 (eleven colonies were picked).
Gel run:
 I55 colony PCR screening. We decided to make glycerol stock for I55-1. Inoculum into 5 ml LB+Amp of the positive culture that was let grow ON at 37°C, 220 rpm.
Miniprep and quantification for I73-1/2/3/4/5/6:
- I73-1: 154 ng/ul
- I73-2: 177 ng/ul
- I73-3: 154,7 ng/ul
- I73-4: 145,5ng/ul
- I73-5: 152,6 ng/ul
- I73-6: 154,2 ng/ul
1,5 hours E-P digest (for screening) and gel run
 I70-1/2/3/4/5 I71-1/2/3/4/5 I736/1/2/3/4/5 E-P A9_4C5E-P A99_4C5E_P screening. As you can see they are (except for I73-6) all positive. So we stocked I73-1 and stored it at -80°C.
Transformation of I74 and INTEIN_C3 (1ul) in E. coli TOP10. They were plated on LB+Amp100 and LB+Cm34 agar plates respectively. They were let grow ON at +37°C.
Inoculum of I55-1 into 5 ml LB+Amp. ON growth at +37°C, 220 rpm.
October, 3rd
Glycerol stock for:
Pick of a single non-red colony of INTEIN_C3 and inoculum into 5 ml LB+Cm34. Sample was let grow ON at +37°C, 220rpm.
Miniprep and quantification of:
- I55-1: 57,4 ng/ul
- I55-1bis: 79,6 ng/ul
I55-1bis was digested E-S for about three hours and than gel run/cut.
Colony PCR for I74 (six colonies were picked).
Gel run for PCR samples and I55 (E-S) and cut for this one.
 I74 screening and I55 E-S cut. Gel extraction and quantification:
Ligation of I55 (E-S) with already available DNA:
- I75: I55 (E-S) + I37 (E-X)
- I77: I55 (E-S) + I57 (E-X)
Inoculum of I35 (last quantification after gel extraction was too poor; we will digest it again E-P) and I54 into 5 ml LB+Amp: ON growth at +37°C, 220 rpm.
|
|
 "
"









