Team:Stockholm/24 September 2010
From 2010.igem.org
(Difference between revisions)
m |
|||
| Line 1: | Line 1: | ||
| + | {{Stockholm/Top2}} | ||
| + | |||
==Andreas== | ==Andreas== | ||
===Plasmid prep=== | ===Plasmid prep=== | ||
| Line 162: | Line 164: | ||
'''Results'''<br /> | '''Results'''<br /> | ||
Seemingly correct bands for samples 1, 3, 4, 5 and 8. More unsure results for 6 and 7, while 2 seems to contain more than one insert, or has been digested at several places. | Seemingly correct bands for samples 1, 3, 4, 5 and 8. More unsure results for 6 and 7, while 2 seems to contain more than one insert, or has been digested at several places. | ||
| + | |||
| + | {{Stockholm/Footer}} | ||
Revision as of 11:06, 26 October 2010
Contents |
Andreas
Plasmid prep
From 23/9 ON cultures
Omega E.Z.N.A. Plasmid Miniprep kit I.
- New plasmid prep buffers
| DNA concentration | ||
|---|---|---|
| Sample | Conc [ng/μl] | A260/A280 |
| pSB1A2.RBS.yCCS 3 | 195.5 | 1.81 |
| pSB1A2.RBS.yCCS 4 | 165.7 | 1.85 |
| pEX.N-TAT⋅SOD⋅His 3† | 60.00 | 1.83 |
| pEX.N-TAT⋅SOD⋅His 4† | 90.00 | 1.73 |
| pSB1K3.N-TAT⋅SOD⋅His 4 | 130.5 | 1.84 |
| pSB1K3.N-TAT⋅SOD⋅His 5† | 60.00 | 1.88 |
| pSB1K3.N-Tra10⋅SOD⋅His 5† | 90.00 | 1.93 |
| pSB1K3.N-LMWP⋅SOD⋅His 1 | 258.2 | 1.87 |
| pSB1C3.N-LMWP⋅SOD⋅His 1 | 155.4 | 1.82 |
| pSB1C3.N-LMWP⋅SOD⋅His 4 | 331.9 | 1.80 |
Cloning and assembly
Digestions
[pEX.RFP] = 44.0 ng/μl
| pA.RBS. yCCS (3) X+P | pEX.N-TAT. SH (4) X+P | pK.N-TAT. SH (4) S+P | pK.N-Tra10. SH (5) X+P | pK.N-Tra10. SH (5) S+P | pC.N-LMWP. SH (4) X+P | pC.N-LMWP. SH (4) S+P | pEX.RFP X+P | |
|---|---|---|---|---|---|---|---|---|
| 10X FastDigest buffer | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 |
| DNA (1 μg) | 5 | 12.5 | 8 | 12.5 | 12.5 | 4 | 4 | 22 |
| dH2O | 11 | 3.5 | 7 | 3.5 | 3.5 | 12 | 12 | 3 |
| FD XbaI | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| FD PstI | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| FD SpeI | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| 20 μl | 20 μl | 20 μl | 20 μl | 20 μl | 20 μl | 20 μl | 30 μl |
- Incubation: 37 °C, 1 h
Gel verification
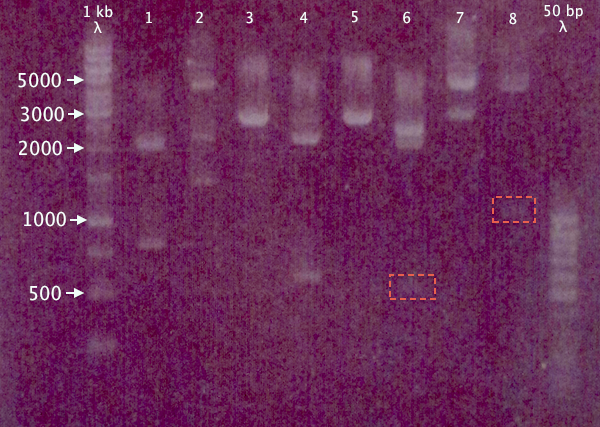
Due to the risk of pSB1A2.RBS.yCCS and pEX.N-TAT⋅SOD⋅His having previously been mixed up, a gel was run on the digested samples (after 15 min incubation) to verify the excised insert size. Remaining samples were also run on the gel to verify digestion.
1 % agarose, 120 V
Expected bands
- pSB1A2.RBS.yCCS X+P: 806 bp, 2061 bp
- pEX.N-TAT⋅SOD⋅His X+P: 558 bp, 4453 bp
- pSB1K3.N-TAT⋅SOD⋅His S+P: ≈2730 bp
- pSB1K3.N-Tra10⋅SOD⋅His X+P: 588 bp, 2188 bp
- pSB1K3.N-Tra10⋅SOD⋅His S+P: ≈2760 bp
- pSB1C3.N-LMWP⋅SOD⋅His X+P: 567 bp, 2054 bp
- pSB1C3.N-LMWP⋅SOD⋅His S+P: ≈2610 bp
- pEX.RFP X+P: 1095 bp, 4453 bp
Results
Seemingly correct bands for samples 1, 3, 4, 5 and 8. More unsure results for 6 and 7, while 2 seems to contain more than one insert, or has been digested at several places.
 |

|
 |

|
 |

|

|

|
 "
"