Team:Lethbridge/Notebook/Lab Work/May
From 2010.igem.org
Liszabruder (Talk | contribs) |
Liszabruder (Talk | contribs) |
||
| Line 100: | Line 100: | ||
Back to [[Team:Lethbridge/Notebook/Lab_Work|Lab Work]]<br> | Back to [[Team:Lethbridge/Notebook/Lab_Work|Lab Work]]<br> | ||
| - | =May= | + | =<font color="white">May= |
| - | ==May 5/2010== | + | ==<font color="white">May 5/2010== |
(in the lab: JV)<br> | (in the lab: JV)<br> | ||
<b>Objective:</b> Test Restriction Endonucleases for activity (take 2)<br> | <b>Objective:</b> Test Restriction Endonucleases for activity (take 2)<br> | ||
| Line 161: | Line 161: | ||
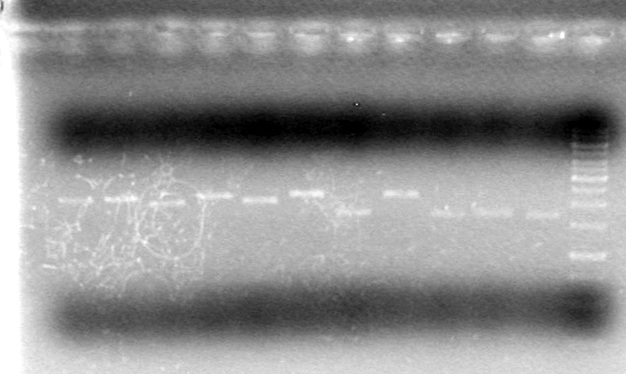
<b>Conclusion:</b> Test other source of SpeI to see if it has any activity.<br><br> | <b>Conclusion:</b> Test other source of SpeI to see if it has any activity.<br><br> | ||
| - | ==May 6/2010== | + | ==<font color="white">May 6/2010== |
(in the lab: KG, AS)<br> | (in the lab: KG, AS)<br> | ||
<b>Objective:</b> To check if the old SpeI enzyme (exp date: March 2011) will cleave plasmid DNA, since we believe the newer SpeI enzyme (exp date: 2012) does not.<br> | <b>Objective:</b> To check if the old SpeI enzyme (exp date: March 2011) will cleave plasmid DNA, since we believe the newer SpeI enzyme (exp date: 2012) does not.<br> | ||
| Line 198: | Line 198: | ||
Placed in -20<sup>o</sup>C freezer of later analysis by agarose electrophoresis<br> | Placed in -20<sup>o</sup>C freezer of later analysis by agarose electrophoresis<br> | ||
| - | ==May 10/2010== | + | ==<font color="white">May 10/2010== |
(in the lab:JV)<br> | (in the lab:JV)<br> | ||
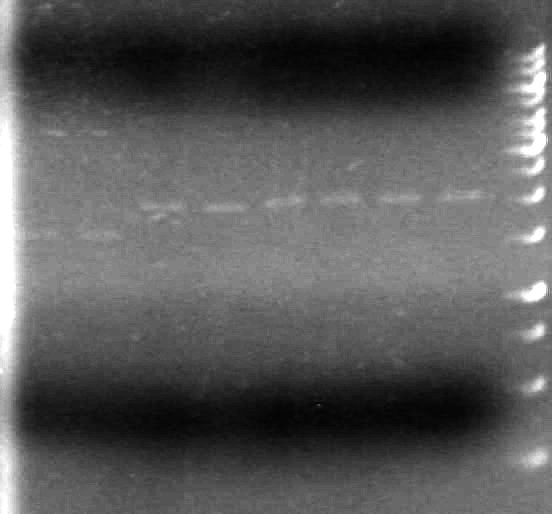
<b>Objective:</b> To analyze the restriction test done by KG and AS on May 6/2010 by agarose electrophoresis<br><br> | <b>Objective:</b> To analyze the restriction test done by KG and AS on May 6/2010 by agarose electrophoresis<br><br> | ||
| Line 230: | Line 230: | ||
Add 500µL of stock ampicillin to 500mL of media<br><br> | Add 500µL of stock ampicillin to 500mL of media<br><br> | ||
| - | ==May 11/2010 Evening== | + | ==<font color="white">May 11/2010 Evening== |
(in the lab: KG, AV, MC, TF, JV, JS)<br> | (in the lab: KG, AV, MC, TF, JV, JS)<br> | ||
<b>Objective:</b> To transform the following plasmids into DH5α <i>E.coli</i> cells. | <b>Objective:</b> To transform the following plasmids into DH5α <i>E.coli</i> cells. | ||
| Line 270: | Line 270: | ||
<li>pTet</li></ul><br> | <li>pTet</li></ul><br> | ||
| - | ==May 12/2010== | + | ==<font color="white">May 12/2010== |
(in the lab: JV)<br> | (in the lab: JV)<br> | ||
<b>Objective: Miniprep of plasmid DNA from transformed cells</b>(JV, AV, HB)<br> | <b>Objective: Miniprep of plasmid DNA from transformed cells</b>(JV, AV, HB)<br> | ||
| Line 390: | Line 390: | ||
Start overnight cultures of cells that grew for plasmid prep and sequencing.<br><br> | Start overnight cultures of cells that grew for plasmid prep and sequencing.<br><br> | ||
| - | ==May 14/2010== | + | ==<font color="white">May 14/2010== |
(in the lab: JV)<br> | (in the lab: JV)<br> | ||
<b>Objective:</b> Quantify pDNA concentration in order to ensure sufficient material for sequence analysis.<br> | <b>Objective:</b> Quantify pDNA concentration in order to ensure sufficient material for sequence analysis.<br> | ||
| Line 462: | Line 462: | ||
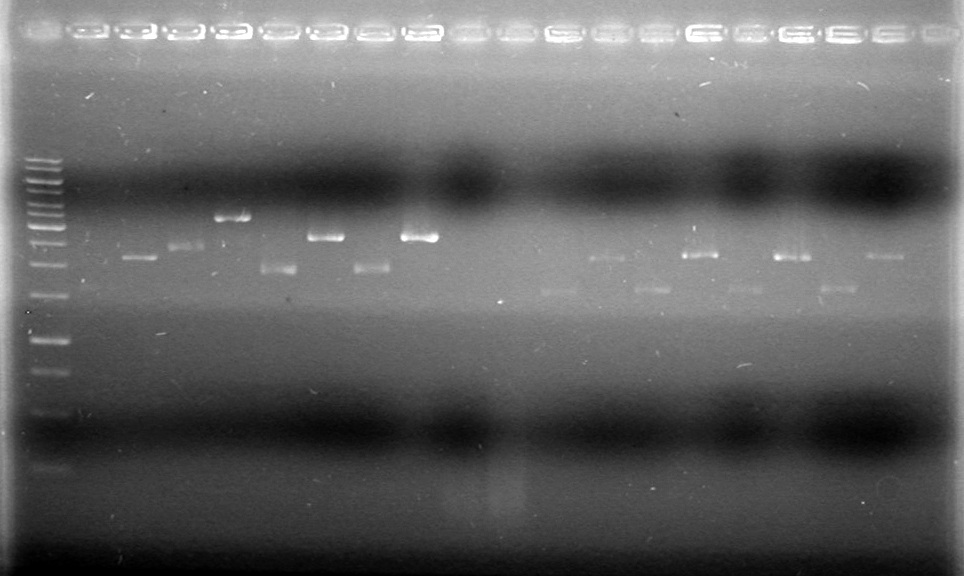
There is plasmid DNA in each sample which, when cut with both the prefix and suffix enzyme, yields a band approximately 2000bp (size of pSB1A3 is 2157bp).<br><br> | There is plasmid DNA in each sample which, when cut with both the prefix and suffix enzyme, yields a band approximately 2000bp (size of pSB1A3 is 2157bp).<br><br> | ||
| - | ==May 17/2010== | + | ==<font color="white">May 17/2010== |
(in the lab: JV, AV)<br> | (in the lab: JV, AV)<br> | ||
Make agar plates with 100µg/mL of ampicillin<br> | Make agar plates with 100µg/mL of ampicillin<br> | ||
| Line 468: | Line 468: | ||
Make 13 x 5mL sterile liquid LB broth<br><br> | Make 13 x 5mL sterile liquid LB broth<br><br> | ||
| - | ==May 17/2010 Evening== | + | ==<font color="white">May 17/2010 Evening== |
(in the lab: TF, AS)<br> | (in the lab: TF, AS)<br> | ||
<b>Objective:</b> To grow cells for future use<br> | <b>Objective:</b> To grow cells for future use<br> | ||
| Line 501: | Line 501: | ||
Only sRBS (D5) and pBad-TetR cells (from transformation plates) grew.<br> | Only sRBS (D5) and pBad-TetR cells (from transformation plates) grew.<br> | ||
| - | ==May 18/2010== | + | ==<font color="white">May 18/2010== |
(in the lab: JV, AV, HB)<br> | (in the lab: JV, AV, HB)<br> | ||
<b>NOTE:</b> Cells from liquid cultures grown last night (May 17/2010) were made into glycerol stocks and placed into the [[Team:Lethbridge/Notebook/Working_Glycerol_Stocks|working glycerol stock box]] as follows:<br> | <b>NOTE:</b> Cells from liquid cultures grown last night (May 17/2010) were made into glycerol stocks and placed into the [[Team:Lethbridge/Notebook/Working_Glycerol_Stocks|working glycerol stock box]] as follows:<br> | ||
| Line 594: | Line 594: | ||
<li>xylE (C4-2007 Box)</li></ul><br> | <li>xylE (C4-2007 Box)</li></ul><br> | ||
| - | ==May 18/2010 Evening== | + | ==<font color="white">May 18/2010 Evening== |
(in the lab: KG)<br> | (in the lab: KG)<br> | ||
<b>Objective:</b> Restrict pLacI, sRNS, sRBS-Lumazine Synthase-dt out of plasmid then ligase pLacI and sRBS also pLacI sRBS-Lumazine Synthase-dt.<br> | <b>Objective:</b> Restrict pLacI, sRNS, sRBS-Lumazine Synthase-dt out of plasmid then ligase pLacI and sRBS also pLacI sRBS-Lumazine Synthase-dt.<br> | ||
| Line 658: | Line 658: | ||
Begin room temperature incubation at 8:25pm. | Begin room temperature incubation at 8:25pm. | ||
| - | ==May 19/2010== | + | ==<font color="white">May 19/2010== |
(in the lab:JV)<br> | (in the lab:JV)<br> | ||
| Line 692: | Line 692: | ||
Reactions ran for 1 hour at 37<sup>o</sup>C.<br> | Reactions ran for 1 hour at 37<sup>o</sup>C.<br> | ||
| - | ==May 20/2010== | + | ==<font color="white">May 20/2010== |
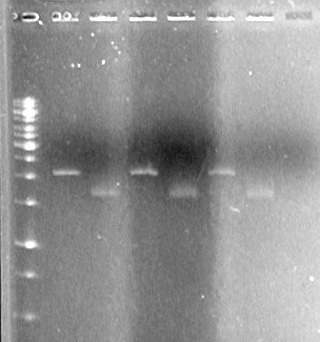
(in the lab:JV, AV)<br> | (in the lab:JV, AV)<br> | ||
| Line 729: | Line 729: | ||
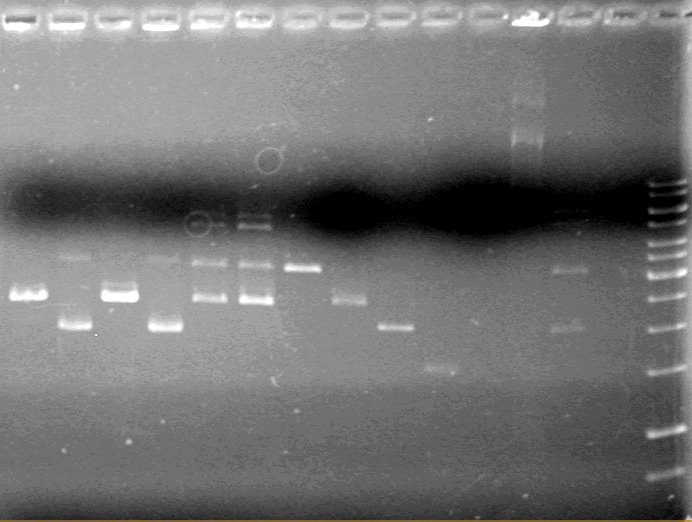
[[image:100520AV.JV-Plasmid Check.JPG|200px|none]] | [[image:100520AV.JV-Plasmid Check.JPG|200px|none]] | ||
| - | ==May 20/2010 Evening== | + | ==<font color="white">May 20/2010 Evening== |
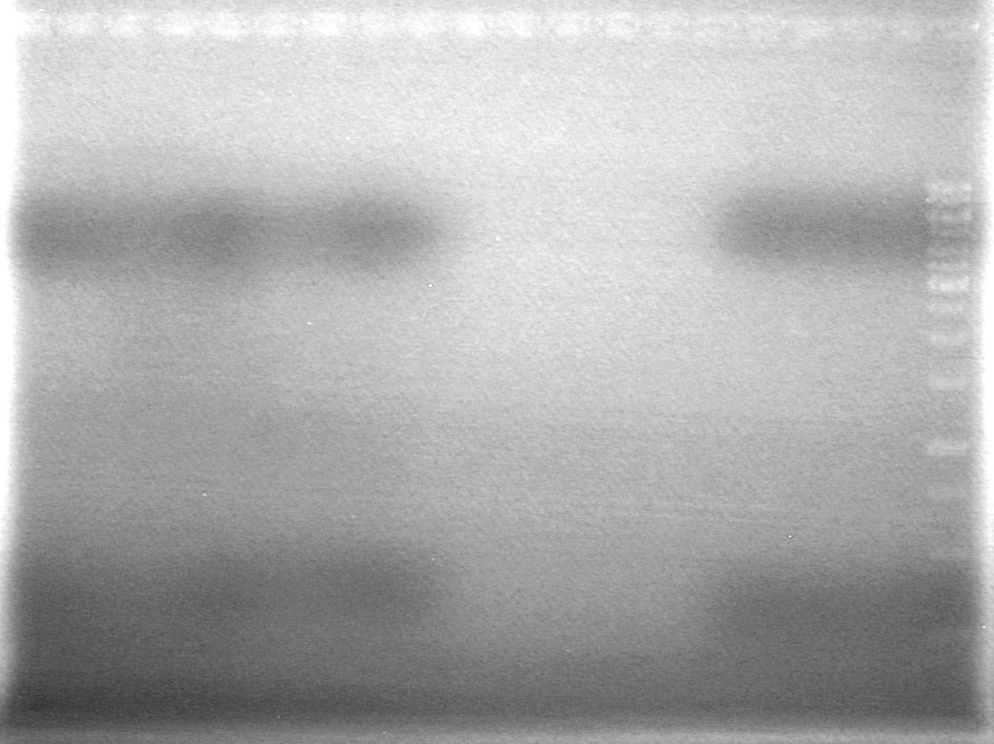
(in the lab:JS, AS)<br> | (in the lab:JS, AS)<br> | ||
| Line 767: | Line 767: | ||
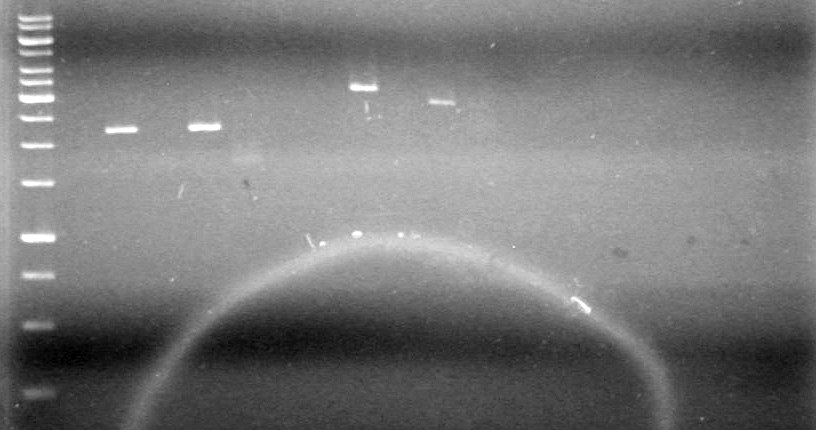
[[image:100520JS1.JPG|200px|none]] | [[image:100520JS1.JPG|200px|none]] | ||
| - | ==May 25/2010== | + | ==<font color="white">May 25/2010== |
(in the lab:JV, HB, AV)<br> | (in the lab:JV, HB, AV)<br> | ||
| Line 781: | Line 781: | ||
<b>NOTE</b> (June 1/2010; AS): Likely that the problem here was the method used to heat shock the cells. Cells were heat shocked in a heat plate, with incomplete contact of the heating surface, causing inefficient transfer of heat to cells for shock and movement of DNA into competent cells. | <b>NOTE</b> (June 1/2010; AS): Likely that the problem here was the method used to heat shock the cells. Cells were heat shocked in a heat plate, with incomplete contact of the heating surface, causing inefficient transfer of heat to cells for shock and movement of DNA into competent cells. | ||
| - | ==May 25/2010 Evening== | + | ==<font color="white">May 25/2010 Evening== |
(in the lab: AV, KG)<br> | (in the lab: AV, KG)<br> | ||
| Line 920: | Line 920: | ||
Removed at 9:30pm<br> | Removed at 9:30pm<br> | ||
| - | ==May 26/2010== | + | ==<font color="white">May 26/2010== |
(In the lab: JV)<br> | (In the lab: JV)<br> | ||
<b>Objective:</b> Purify plasmid DNA (via mini-prep) of the cells grown last night.<br> | <b>Objective:</b> Purify plasmid DNA (via mini-prep) of the cells grown last night.<br> | ||
| Line 941: | Line 941: | ||
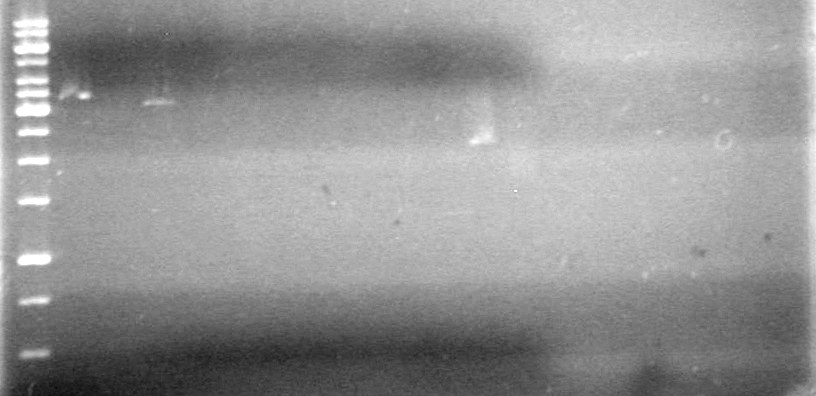
<b>Results:</b> Will restrict and run agarose gels tomorrow.<br> | <b>Results:</b> Will restrict and run agarose gels tomorrow.<br> | ||
| - | ==May 27/2010== | + | ==<font color="white">May 27/2010== |
(In the lab: JV, AV)<br> | (In the lab: JV, AV)<br> | ||
| Line 1,039: | Line 1,039: | ||
AV quantified each pDNA stock in the [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids box]] by measuring the A<sub>260</sub> of a 1:100 dilution of the pDNA samples in a UV cuvette. Results are posted on the [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids]] page.<br> | AV quantified each pDNA stock in the [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids box]] by measuring the A<sub>260</sub> of a 1:100 dilution of the pDNA samples in a UV cuvette. Results are posted on the [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids]] page.<br> | ||
| - | ==May 31/2010 - Evening== | + | ==<font color="white">May 31/2010 - Evening== |
<b>Objective:</b> Transform recent ligation reactions that didn't work the first time around.<br> | <b>Objective:</b> Transform recent ligation reactions that didn't work the first time around.<br> | ||
<b>Method:</b> Use [[Team:Lethbridge/Notebook/Protocols|competent cell transformation]] protocol to transform the following ligation products:<br> | <b>Method:</b> Use [[Team:Lethbridge/Notebook/Protocols|competent cell transformation]] protocol to transform the following ligation products:<br> | ||
 "
"