|
SEPTEMBER: WEEK 3
September, 13th
Unfortunately we discovered we tried to ligate I32 (E-S) to I37 (X-P) instead of I37 (E-X) ;-(
So this step will be repeated.
Trasformation of ligations
into E. coli DH5-alpha.
Inoculum of
into 5 ml LB+Amp for ligations of the following day.
Inoculum from single colony of MG42 and MC43 in 5 ml LB+Cm12.5, than ON at 37°C.
September, 14th
Finally Mr.Gene sent us our YEAST: a little difficult to pick up the package but at the end we succeeded!
I52, I54, I55, I56, I57, I58 plates showed in general few colonies; I52 and I5X showed very few colonies (<=5). Very strange (mumble mumble...), they will be screened ASAP (stored at +4°C).
Miniprep and quanfification of:
- I26: 69,7 ng/ul
- I31: 89,5 ng/ul
- I34: 147,6 ng/ul
3-hours digestion:
- I26: XbaI-PstI (Insert)
- I31: EcoRI-SpeI (Insert)
- I34: E-X (Vector)
Gel run/cut of samples (I26 and I31 insert bands were very very soft)
 Gel run/cut for digested I26, I31 and I34. and gel extraction:
- I26 (X-P): 2,6 ng/ul
- I31 (E-S): 1,4 ng/ul
- I34 (E-X): ng/ul
We already had digested DNA so we could perform new ON ligations
- I59: I21 (E-S) + I34 (E-X)
- I60: I20 (S-P) + I26 (X-P)
- I61: I31 (E-S) + I37 (E-X)
and repeat
- I53: I32 (E-S) + I37 (E-X)
Inoculum of I47, I48, I49 into 5 ml LB+Amp for TECAN test of the following day.
Screening PCR on 3 colonies picked from MC42 and MC43 respectively.
Two method were used: our standard PCR picking a colony from the plate and using it for the PCR and a different protocol picking the colony and treating it with a 95°C 5 min step before the PCR.
The results were the follow:
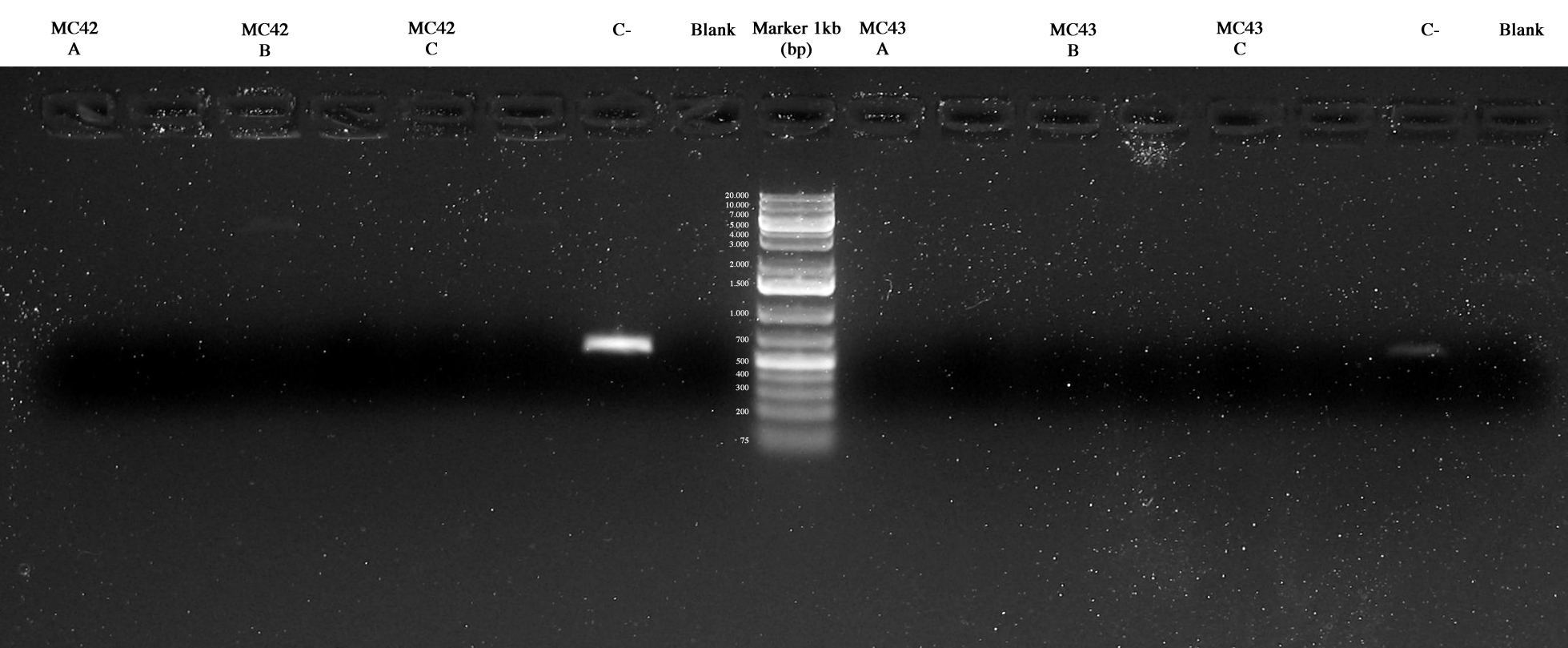 MC42A/B/C, Cneg, blank, MC43A/B/C, Cneg, blank. Negative controls were ok and maybe MC42_C and MC43_C were ok too but further investigations were necessary.
Tecan Test was performed on prepared samples, after the usual protocol (dilution, medium change and dilution 1:1000).
September, 15th
I47, I48, I49 cultures were diluted 1:100 into 5ml LB+Amp.
In the afternoon all cultures were synchronized to O.D. 0,02 and Tecan Test was started to check GFP production by these BioBricks.
Transformation of I 53, I59, I60, I61 ligations into E. coli DH5-alpha. They were plated on LB+Amp agar plates and let grow ON at 37°C.
September, 16th
All plates showed colonies, so we could perform screening through "colony PCR" for ligations:
- I52
- I53
- I54
- I55
- I56
- I57
- I58
- I59
- I60
- I61
For each plate we picked X colonies that were also inoculated into 1 ml LB+Amp in order to be ready to make glycerol stock of positive ones.
Agarose gel was prepared and samples loaded and run:
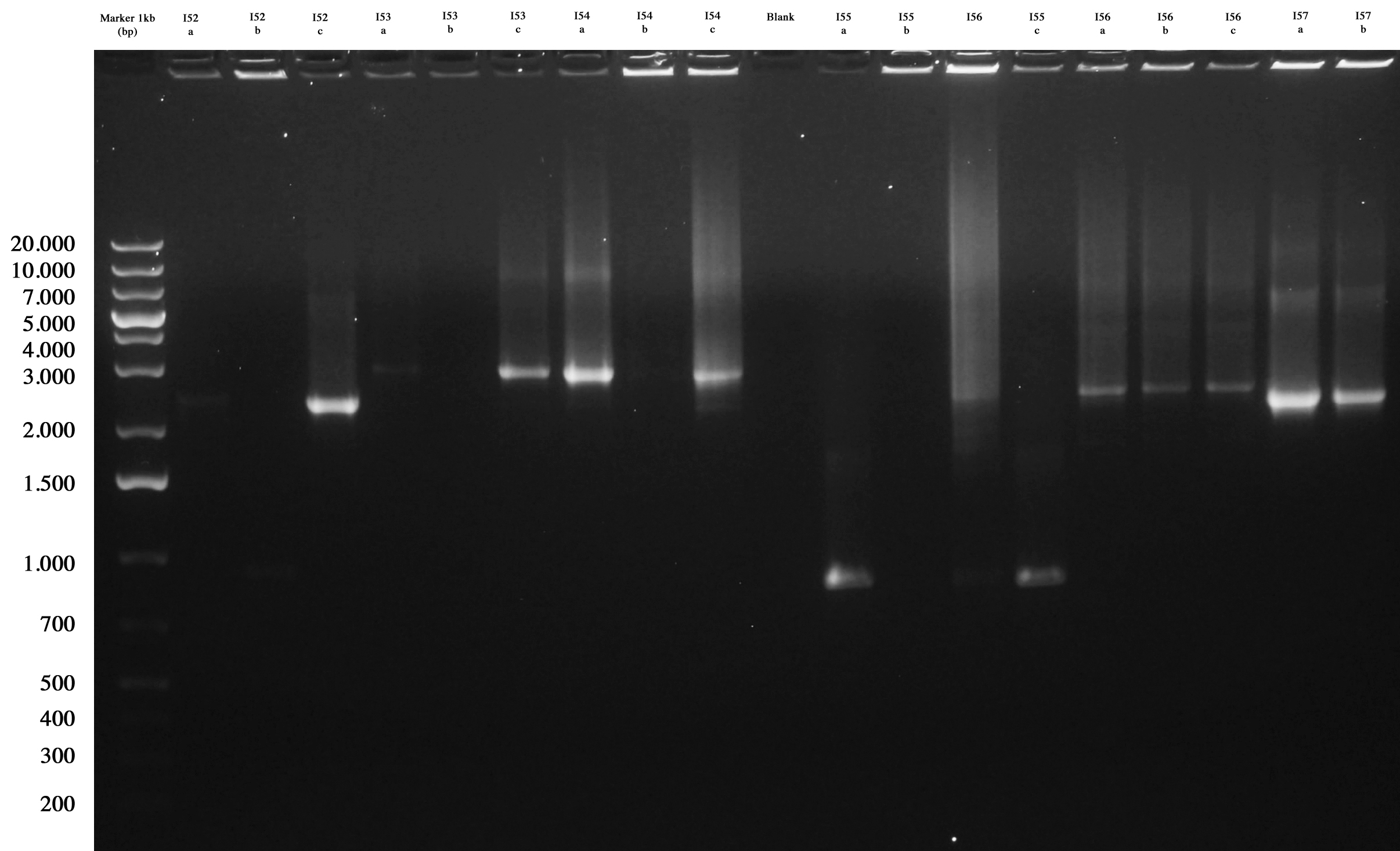 Screening gel run for ligations I52..I57. |  Screening gel run for ligations I58..I61. |
As you can see I53a/c, I54a, I56a/b/c, I57a/b are positive. So we decided to make glycerol stocks for I53c, I54a, I56a and I57a. I52 and I55 were all negative. We would have performed colony PCR again. Probably all colonies I58..I60 were positive but their length was a little longer than expected. So we decided to further screen them through digestion.
I58a/b/c, I59a/b/c, I60a/b/c and I61a/b/c were let grow ON at 37°C in order to perform miniprep the following day.
Tecan Test was performed on prepared samples, after the usual protocol (dilution, medium change and dilution 1:1000).
September, 17th
Miniprep and Nanodrop quantification for:
- I58a: 127,5 ng/ul
- I58b: 108,9 ng/ul
- I58c: 100,1 ng/ul
- I59a: 161,6 ng/ul
- I59b: 268,8 ng/ul
- I59c: 214,3 ng/ul
- I60a: 151,6 ng/ul
- I60b: 149,7 ng/ul
- I60c: 157,6 ng/ul
- I61a: 147,1 ng/ul
- I61b: 138,3 ng/ul
- I61c: 125,3 ng/ul
Digestion (for screening) and gel extraction of positives (for next ligation step) for:
- I58a/b/c: EcoRI-SpeI
- I59a/b/b: XbaI-PstI
- I60a/b/c: E-S
Digestion (only screening) for
Agarose gel was prepared and samples loaded/run/cut (were necessary)
 Screening and gel extraction for positive I58, I59, I60. Screening for I60. As you can see...
September, 18th
|
|
 "
"





