Team:Calgary/23 July 2010
From 2010.igem.org
H.dastidar (Talk | contribs) |
|||
| Line 1: | Line 1: | ||
{{CalgaryNotebookTemplate| | {{CalgaryNotebookTemplate| | ||
| - | |||
| - | |||
| - | |||
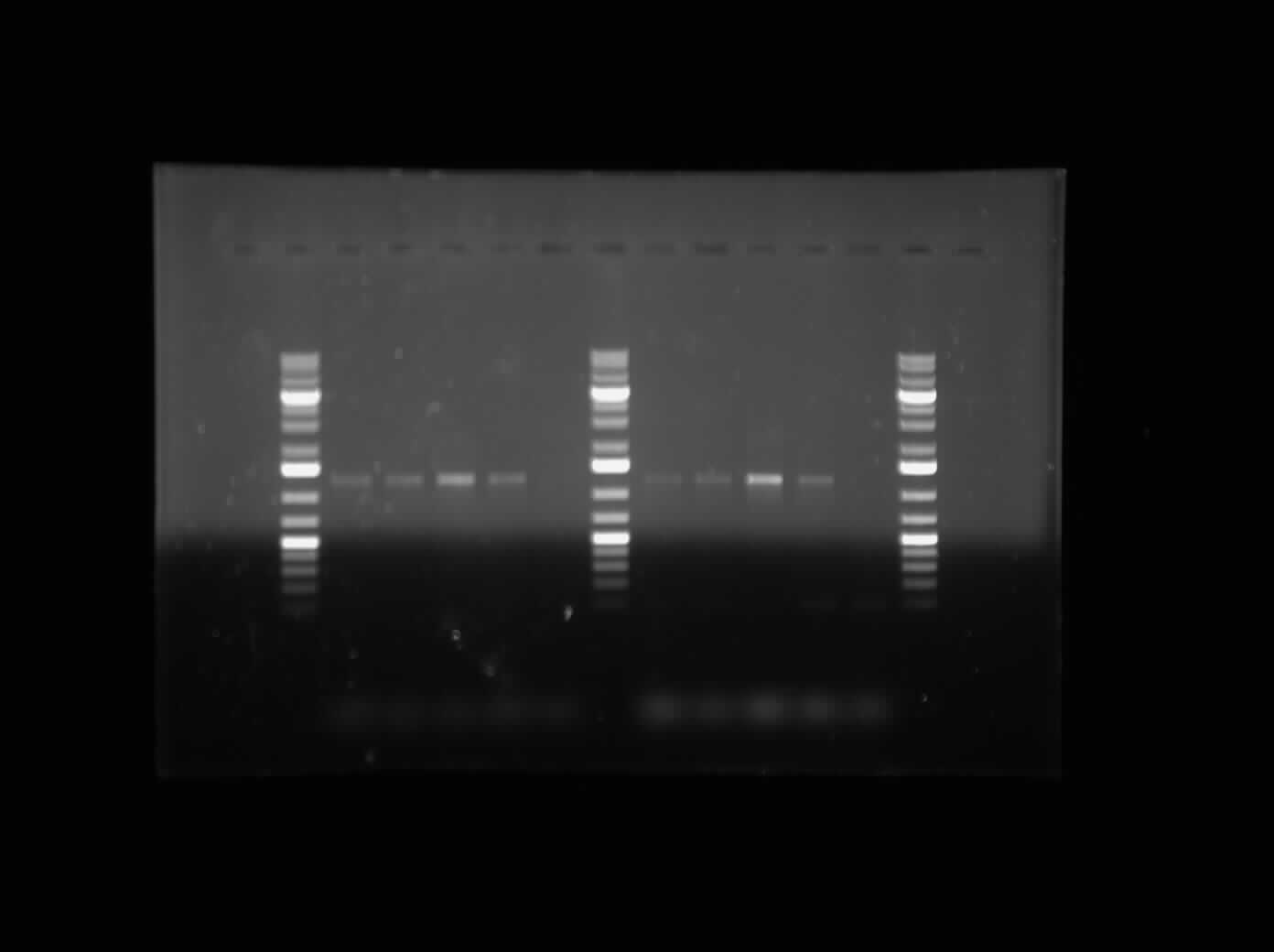
[[Image:07.23.2010. MalEGeneSpec.jpg|thumb|400px|Gel electrophoresis of Raida's PCR products: Mal-E Gene specific primers]] | [[Image:07.23.2010. MalEGeneSpec.jpg|thumb|400px|Gel electrophoresis of Raida's PCR products: Mal-E Gene specific primers]] | ||
| Line 8: | Line 5: | ||
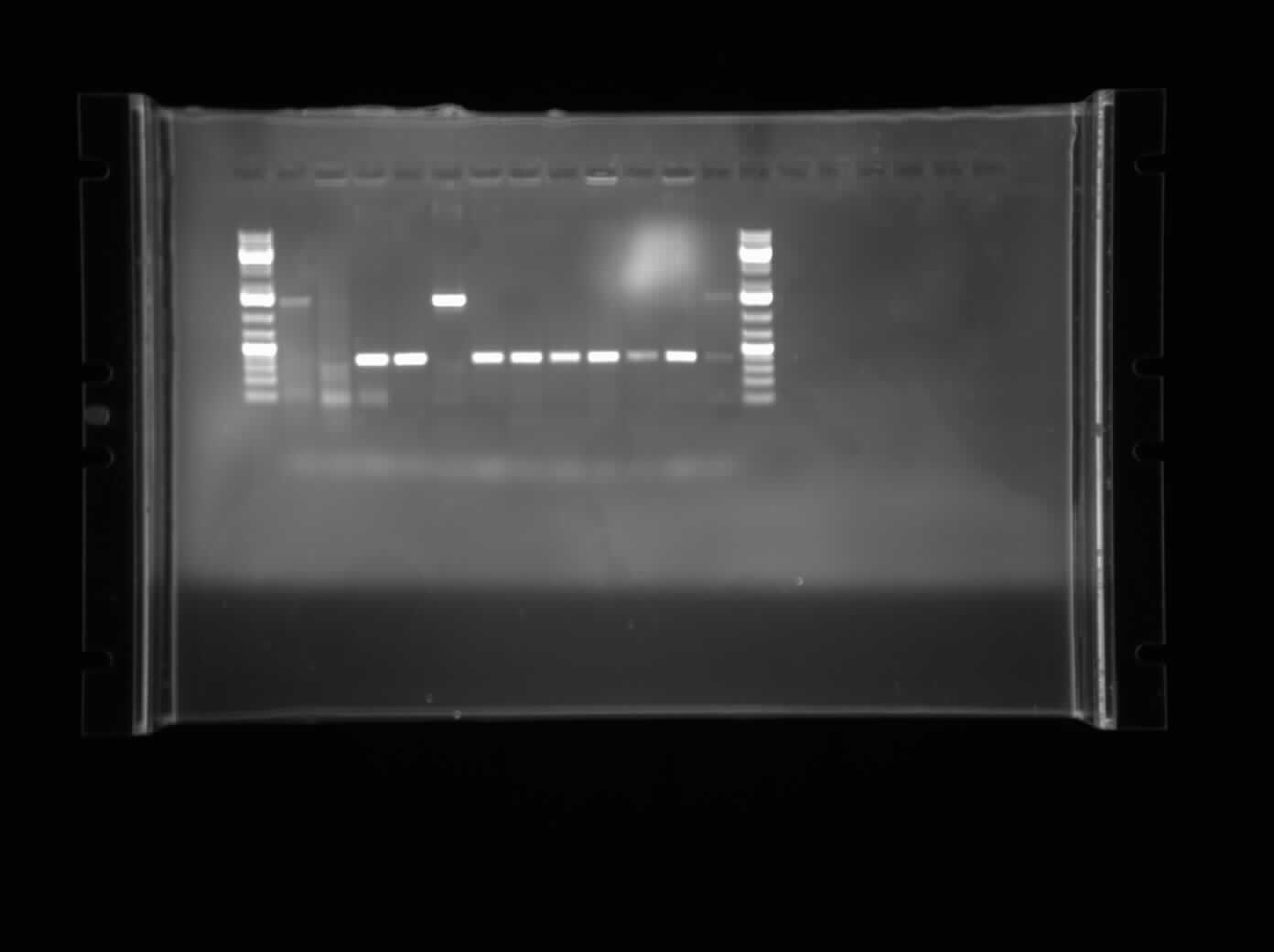
[[Image:07.23.2010.himika-I0500-B0034-PSB1AC3.jpg|thumb|400px|Himika's colony PCR of I0500-B0034 construction in pSB1AC3. The bands were expected to be around 1400 bp and they turned out to be as expected. Lane 2 and 3 will be restreaked, plasmid prepped and sequenced.]] | [[Image:07.23.2010.himika-I0500-B0034-PSB1AC3.jpg|thumb|400px|Himika's colony PCR of I0500-B0034 construction in pSB1AC3. The bands were expected to be around 1400 bp and they turned out to be as expected. Lane 2 and 3 will be restreaked, plasmid prepped and sequenced.]] | ||
| + | |||
| + | '''Friday July 23, 2010''' | ||
| + | |||
<u> Raida </u> | <u> Raida </u> | ||
The first thing I did this morning was run a Gel Electrophoresis of my Mal-E Gene Specific Primer Testing PCR products. I ran it on a 1% gel at 100 V for an hour. Please refer to the image to the side. As it can be seen, the PCR results are positive. All the bands are near the 1200 bp band size, which indicates that it is the Mal-E gene. Furthermore, Lane 7 and Lane 13 show no band because this was the DNA that had the deleted sequence. So the fact that there is no band there is an indication of the fact that the '''MalE primer is functional and only amplified the MalE gene as it was supposed to'''. Further testing will be done to assure that the band shows the MalE Gene. | The first thing I did this morning was run a Gel Electrophoresis of my Mal-E Gene Specific Primer Testing PCR products. I ran it on a 1% gel at 100 V for an hour. Please refer to the image to the side. As it can be seen, the PCR results are positive. All the bands are near the 1200 bp band size, which indicates that it is the Mal-E gene. Furthermore, Lane 7 and Lane 13 show no band because this was the DNA that had the deleted sequence. So the fact that there is no band there is an indication of the fact that the '''MalE primer is functional and only amplified the MalE gene as it was supposed to'''. Further testing will be done to assure that the band shows the MalE Gene. | ||
| + | |||
<u>Himika</u> | <u>Himika</u> | ||
| Line 24: | Line 25: | ||
I also restreaked the two colonies on AC plates and left it in the incubator at 20 C. I also made overnight cultures of the same colonies which will be taken out of the shaker on saturday 24 July, 2010. | I also restreaked the two colonies on AC plates and left it in the incubator at 20 C. I also made overnight cultures of the same colonies which will be taken out of the shaker on saturday 24 July, 2010. | ||
| + | |||
| + | |||
| + | |||
}} | }} | ||
Revision as of 21:30, 23 July 2010

Friday July 23, 2010
Raida
The first thing I did this morning was run a Gel Electrophoresis of my Mal-E Gene Specific Primer Testing PCR products. I ran it on a 1% gel at 100 V for an hour. Please refer to the image to the side. As it can be seen, the PCR results are positive. All the bands are near the 1200 bp band size, which indicates that it is the Mal-E gene. Furthermore, Lane 7 and Lane 13 show no band because this was the DNA that had the deleted sequence. So the fact that there is no band there is an indication of the fact that the MalE primer is functional and only amplified the MalE gene as it was supposed to. Further testing will be done to assure that the band shows the MalE Gene.
Himika
Today I ran a colony PCR of 11 of the colonies from the plates last night which was I0500-B0034 plasmid switched into pSB1AC3. Most of the colony PCRs seem to work, although the bands are faint. Primers used:
- Bbk_CP_F
- Bbk_CP_R
These two primers anneal 100 bp upstream and downstream of the biobrick prefix and suffix. The expected size of the bands was 1400 bp which was exactly what was seen in the gel. I have decided to forward with B1,C1 which are lanes 2 and 3 respectively.
I also restreaked the two colonies on AC plates and left it in the incubator at 20 C. I also made overnight cultures of the same colonies which will be taken out of the shaker on saturday 24 July, 2010.
 "
"