Lab notes (7/19 - 7/25)
Group: Flagella
Annealing of the two mutated strands of FlhDC
Experiment done by: Sheila
Date: July 19th
Protocol: CP1.1
Method: PCR of the two mutated strands of the FlhDC operon
Notes: To samples were run at two different temperatures: 56,1˚C and 64,5˚C respectively.
Polymerase used: Pfu
Primers used: None, as the two strands are supposed to anneal to each other
Results:
Start date: 19/07 End date: 19/07
Methods: Gel extraction, nanodrop
Protocol:DE1.1
Experiment done by:Maria
Notes:
70uL of flhD/C PCR product from Amplification of flhD/C was loaded onto a 1.5 agarose gel and extracted according to protocol.
DNA was eluted in 20uL elution buffer.
Results:
Nanodrop:
Sample ID
|
ng/uL
|
260/280
|
260/230
|
flhDC 1
|
19.82
|
2.43
|
0.08
|
flhDC 2
|
25.24
|
2.22
|
0.12
|
Analysis:
Nanodrop measurements indicated a possible contamination. However the DNA was pooled and used for Digestion
--Tipi 17:22, 19 July 2010 (UTC)
Digestion of flhD/C and pSB1A2 with EcoRI SpeI
Start date: 19/07 End date: 19/07
Methods: Digestion, Gel electrophoresis
Protocol:RD1.1
Experiment done by:Maria
Notes:
purified pSB1C3 (tube 18 blue) and flhD/C from Gel extraction was digested with EcoRI and SpeI.
Restriction mixture:
|
flhD/C
|
pSB1C3
|
H2O
|
30uL
|
24uL
|
EcoRI
|
4uL
|
2uL
|
SpeI
|
4uL
|
2uL
|
FD green buffer
|
8uL
|
4uL
|
DNA
|
38uL
|
10uL
|
total vol.
|
84
|
42
|
samples were loaded onto a 1.5 agarose gel. Gene ruler red were used as marker.
Results:
Gel electrophoresis:
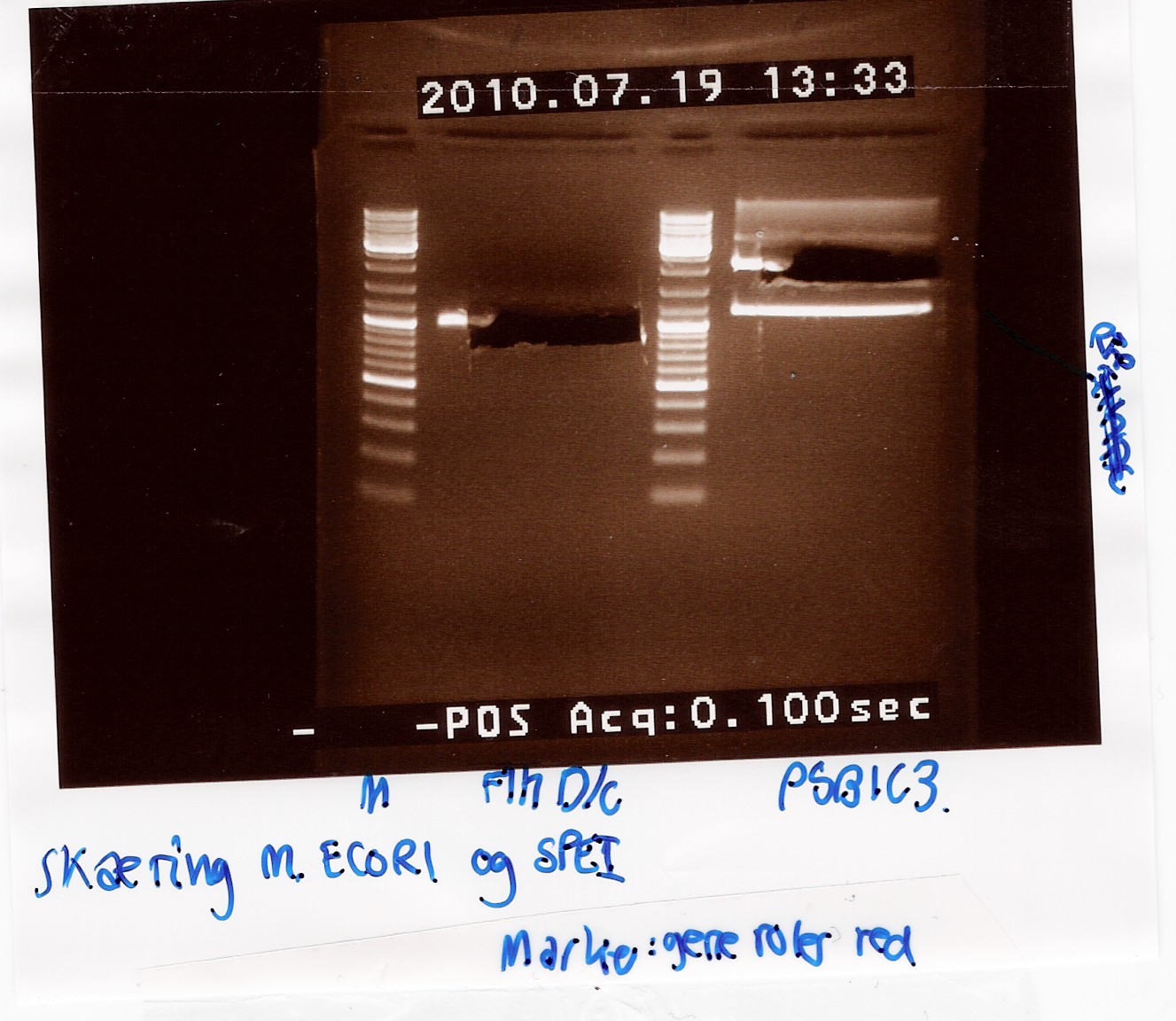
Analysis:
In lane 2 containing pSB1C3 2 bands are detected indicating a succesful digestion of the plasmid (the band at 1000 bp corresponds to J04450). A succesful digestion of the flhD/C cannot be concluded from the gel. However both bands was excised and extracted from gel (Gel extraction)
--Tipi 17:42, 19 July 2010 (UTC)
Gel extraction of digested flhD/C and pSB1C3
Start date: 19/07 End date: 19/07
Methods: Gel extraction, nanodrop
Protocol:DE1.2
Experiment done by:Maria
Notes:
Digested flhD/C and pSB1C3 from Digestion were extracted from gel according to protocol.
DNA was eluted in 20uL H20.
Results:
Nanodrop:
Sample ID
|
ng/uL
|
260/280
|
260/230
|
flhD/C
|
18.18
|
4.59
|
0.18
|
pSB1C3
|
14.30
|
1.89
|
0.04
|
Analysis:
nanodrop measurements indicated contamination. However both samples were used for Ligation.
--Tipi 17:57, 19 July 2010 (UTC)
Ligation of flhD/C and pSB1C3
Start date: 19/07 End date: 20/07
Methods: Ligation
Protocol:LG1.2
Experiment done by:Maria
Notes:
3 ligation reactions was prepared.
|
LG1
|
LG2
|
LG3
|
10x T4 ligase buffer
|
2uL
|
2uL
|
2uL
|
flhD/C
|
5uL
|
5uL
|
5uL
|
pSB1C3
|
1uL
|
2.8uL
|
5uL
|
H20
|
11uL
|
9.2uL
|
7uL
|
T4 ligase
|
1uL
|
1uL
|
1uL
|
Ligation mixtures were not run on gel but were directly used for transformation
--Tipi 18:15, 19 July 2010 (UTC)
Group: Photosensor
Group: Retinal
Transformation of K081005 in pSB1A2 (constitutive promoter and RBS combined),R0011 in pSB1A2, pSB3C5 w. J04450 and pSB3T5 w. J04450 in Top 10 E.Coli
Start date: 19/07 End date: 20/07
Methods: ON culture, making competent cells, transformation
Protocol:CC1.1 TR1.1
Experiment done by: Maria
Notes:ON colony was made of 110 ml lb medium inoculated with a top10 coloni.
Time
|
Optical density
|
8:12
|
2.9
|
8:17
|
0.02
|
9:17
|
0.035
|
10:17
|
0.204
|
10:40
|
0.380
|
10:50
|
0.49
|
pSB1A2 w. R0011 and pSB1A2 w. K081005 was plated with 150uL on plates containing LA, LA + Amp, LA + Tetracycline, LA + Chloramphenicol and LA + Kanamycine.Upconcentration of these samples was not made. pSB3T5 w. J04450 and pSB3C5 w. J04450 was plated according to protocol
Results:
Analysis:
Both pSB3T5 and pSB3C5 was succesfully transformed, and ON cultures with appropiate antibiotics were made for mini-prep.
For pSB1A2 w. R0011 only 6 colonies was observed on the LA+amp plate.
For pSB1A2 w. K081005 only 4 colonies was observed on the LA+amp plate.
All 10 colonies were used for coloni PCR.
--
Tipi 16:33, 19 July 2010 (UTC)
Transformation of flhD/C in pSB1C3 and test plasmid in Top 10 E.Coli
Start date: 20/07 End date: 19/07
Methods: ON culture, making competent cells, transformation
Protocol:CC1.1 TR1.1
Experiment done by: Maria
Notes:
Ligated flhD/C from Ligation and test plasmid from Whatman was transformed.
ON colony was made of 25 ml lb medium inoculated with a top10 coloni.
Time
|
Optical density
|
8:17
|
3.5
|
8:20
|
0.02
|
9:15
|
0.035
|
10:30
|
0.222
|
10:52
|
0.350
|
11:05
|
0.52
|
Compotent cells were washed using 10mL 50mM CaCl2.
3 parallel transformations were carried out for L1, L2 and L3 respectively (see
Ligation). L1.1, L1.2, L2.1, L2.2 and L3.1 were transformed using compotent cells from 19/7 (
Compotent cells).
Prior to transformation test plasmid was washed with 10xTE pH 8.0.
--
Tipi 15:19, 20 July 2010 (UTC)
 "
"
