Team:Heidelberg/Project/Capsid Shuffling/ViroBytes
From 2010.igem.org
(→Introduction) |
(→Protocols) |
||
| Line 123: | Line 123: | ||
==Protocols== | ==Protocols== | ||
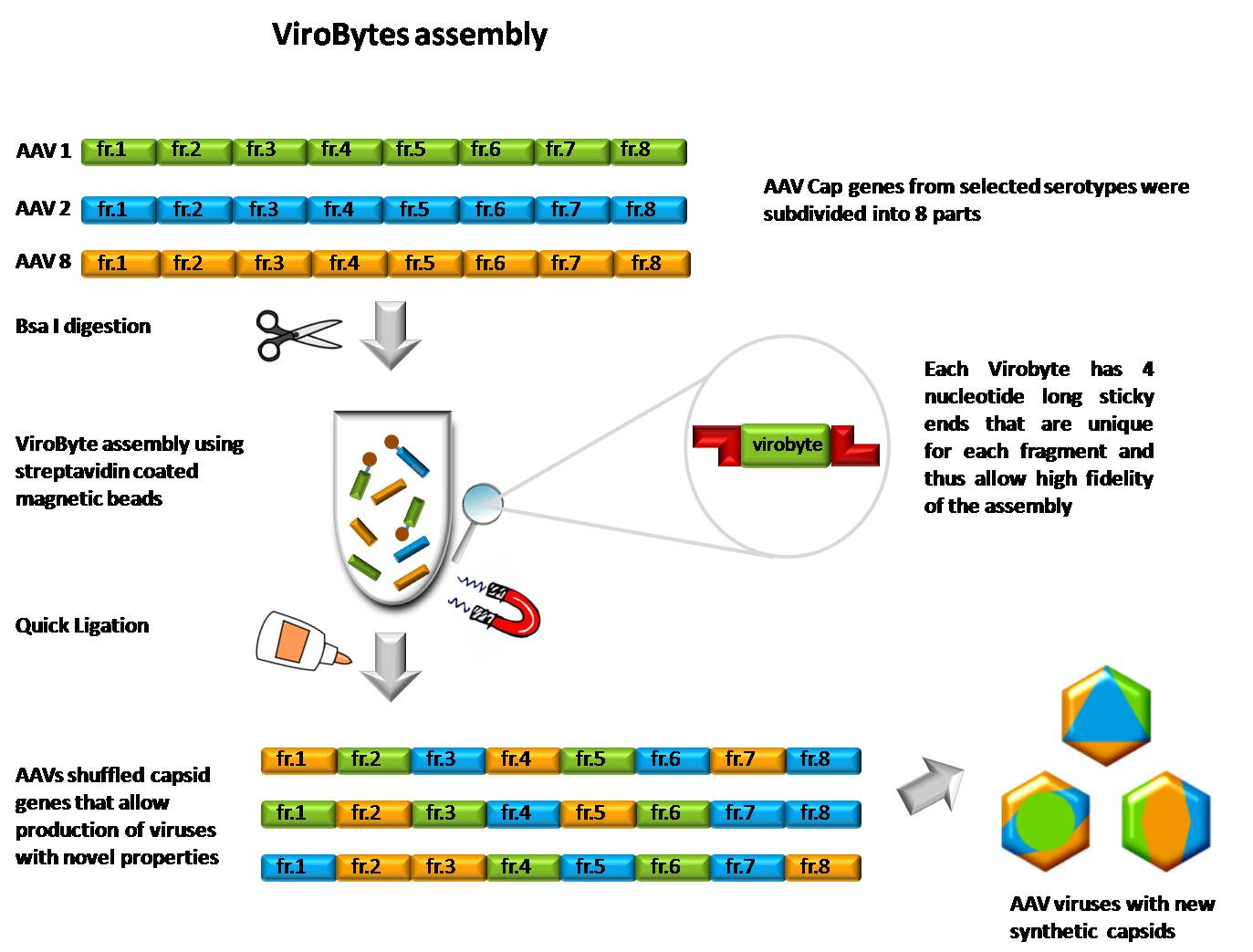
| + | **'''ViroByte construction''' | ||
| - | + | *perform ViroByte PCR to obtain desired fragments with complementary ends | |
| + | *digestion with Bsa1 | ||
| + | |||
| + | **'''Anchor''' | ||
| + | |||
| + | *after washing the beads and aspiration of the cleared solution the beads are ready for adding of the Anchor | ||
| + | |||
| + | *add 40ul of the Anchor solution (~200ng/ul) | ||
| + | |||
| + | *resuspend the beads and let incubate at room temperature for 15-20 minutes with occasional flicking in order to maintain the homogeneity of the solution | ||
| + | |||
| + | *applying magnet, wash twice with 80ul of wash/binding buffer (0.5 M NaCl; 20 mM Tris HCl (pH 7.5); 1 mM EDTA) and once with 80ul of 1X T4 ligase buffer | ||
| + | |||
| + | **'''BioByte-like assembly''' | ||
| + | |||
| + | *Bead preparation | ||
| + | |||
| + | *Quickly vortex beads stock solution to obtain homogeneous suspension | ||
| + | |||
| + | *Aliquot 40 µl of the beads into 1.5ml Eppendorf tubes - gently tap the tubes to collect all the beads at the bottom. | ||
| + | |||
| + | *Place the tubes on the magnetic rack (or place a magnet on the side of each tube) and wait for the beads to be cleared out from the solution | ||
| + | |||
| + | *Carefully discard the supernatant whilst beads are still attached to the side wall | ||
| + | |||
| + | *Resuspend beads in 80ul of wash/binding buffer by gentle flicking (vortexing at high speed is not recommended) | ||
| + | |||
| + | *Wash the beads once with 80 µl of 1X T4 Ligase buffer, before addition of ligation premix. | ||
| + | |||
| + | *Add the DNA fragments using the ligation premix: 1) 25 µl of Quick Ligase (2X buffer); 2) Amount of DNA corresponding to 200 ng. The optimal DNA concentration for the ViroByte fragments assembly was experimentally established and was in range of 200 ng per fragment. Using higher and lower DNA concentrations did not give any positive assembly results; 3) Fill up with water to obtain the 50 µl total volume. | ||
| + | |||
| + | *For the ligation procedure the Quick Ligase was used that showed to be more efficient during the experimental procedure in comparison to the ordinary T4 Ligase. Optimal ligation time for the Virobytes ligation was between 15-25 minutes. | ||
| + | |||
| + | *After the last DNA fragment ligation wash the beads once with Wash/Binding buffer. Moreover the DNA should be cleaved from the beads by using HindIII restriction enzyme (or the other one depending on your designed cleavage site). For the restriction a standard protocol for the DNA digestion should be used. | ||
==Notebook== | ==Notebook== | ||
Revision as of 03:08, 28 October 2010

|
|
|||
 "
"