Lab notes (7/12 - 7/18)
Group: Flagella
Exp. 1
Methods: [MiniPrep], NanoDrop and gel electrophoresis
Notes: We used 9 ml ON culture (Lag phase cells), loaded 2ul sample and 8ul agarose loading dye on a 1,5% gel,used a DNA ladder mix (100-10000 nucleotides) as marker
Results: Nanodrop: Tube 1: 30.7 mg/ul Tube 2: 27.4 mg/ul
Gel electrophoresis: No result
--Louch07 15:00, 9 July 2010 (UTC)
Exp. 2
Methods: gel electrophoresis
Notes: We ran another gel electrophoresis on the miniPrep sample form above. But now we loaded 4ul sample and 4ul agarose loading dye on a 1,5% gel,used a DNA ladder mix (100-10000 nucleotides) as marker.
Results: Gel electrophoresis: Bands were detected. The psb3k3 plasmid is 2750bp long and the RFP with generator is 1096 bp, which gives a band at about 4000bp compaired with the marker.
Exp. 3
Methods: [MiniPrep], NanoDrop and gel electrophoresis.
Notes: We used 10 ml af a culture in Log phase (1ml cells from an ON culture + 9ml LB medium, incubated at 37 degrees celcius for 4 hours), loaded 4ul sample and 4ul agarose loading dye on a 1,5% gel,used a DNA ladder mix (100-10000 nucleotides) as marker.
Results: Nanodrop: Tube 1: 38.7 mg/ul Tube 2: 32.4 mg/ul.
Gel electrophoresis: Bands were detected.
--Louch07 17:21, 12 July 2010 (UTC)
Polyferation of FlhDC, FlhD and FlhC genes
Methods: [PCR] and Gel electrophoresis.
Notes: We decided to test our primers on previously purified cromosomal DNA. Examination of the primers showed that the FlhC reverse primer had a melting temperature of only 45˚C. Therefore we decided to run the samples on a gradient PCR. Simultaneously, we prepared 2 extra samples, isolating FlhD and FlhC, respectively. We did this because we wanted to see if our problems were caused because the combined gene-sequence was to long (932bp).
Because we just wanted to test our primers in this PCR, we used Taq polymerase, because although it doesn’t proofread, it is remarkably cheaper than Pfu. On the [http://www.fermentas.com/en/products/all/pcr-qpcr-rt-pcr/standard-pcr/ep028-taq-dna-native Fermentas homepage] we found that the annealing temperature for Taq is Tm-5 , which in this case means 40˚C. However, Taq polymerase is not very effective at temperatures under 50˚C so we designed the gradient to lies between 40 and 55˚C. More specifically we chose the following temperatures:
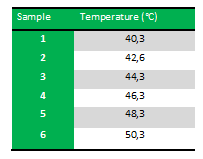
Results: The experiment was succesfull! We could detect FlhDC DNA at temperatures between 42.6˚C and 48.3˚C. FlhD DNA at temperatures between 40.3˚C and 44.3˚C and also between 48.3˚C and 50.3˚C. FlhC DNA at temperatures run between 40.3˚C and 50.3˚C.
--Louch07 10:13, 12 July 2010 (UTC)
Polyferation og FlhDC with Pfu
methods PCR and gel electophoresis
Notes The purified chromosomal DNA was tested with Pfu; a polymerase with proofreading ability. The PCR was run at 44˚C. We decide to run the PCR at this temperature while it is in the middle of the temperature span we successful used in the last experiment.
results Unfortunately the experiment did not give any results on the gel.
Group: Photosensor
Group: Retinal
Colony PCR of K274210 in pSB1A2 (transformation from 08/07)
Start date: 12/07 End date: 12/07
Methods: [Colony PCR]
Protocol: Modified iGEM colony PCR protocol
Notes: MgCl2 was added as a gradient,sample 1-2 contain 2 ul (0,04255), sample 3-5 2,25ul (0,04787). We mistakenly added 30ul (instead of 17ul) premix to sample 6 - 9, so the concentration of MgCl2 is reduced. Sample 6 contains 2,25ul (0,0375), Sample 7-8 2,5ul (0,04167), Sample 9 2,75ul (0,04583).
Gel was loaded with DNA-ladder plus, with the upper marker at 5000bp.
Results:
We found plasmids at the expected 5kbp marker in colonies 1-8.
Transformation of BBa_R0011 and BBa_I0500
Start date: 12/07 End date:
Methods: ON culture; [Competent cells of E.Coli for transformation]; [Transformation of E.Coli].
Protocols: iGEM protocol for competent cells, iGEM protocol for transformation of E.Coli.
Experiment done by: Christian, Maria and LC (Making competent E.Coli)
Date: 12/07
Notes: Everything was done after protocol.
Results:
Analysis:
Experiment done by: Christian, Maria and LC (Transformation of pBad and lacl promoter)
Date: 12/07
Notes: Added 200ul of the un-pelleted cells, instead of 150ul to the agar plates.
Some of the agar dishes were damaged during drying and plating. We tried to use them anyways.
Results:
Analysis:
Mini-prep of pSB1A2 w. B0034, pSB1AK3 w. B0015 and pSB3K3 w. J04450(transformation from 08/07)
Start date: 12/07 End date: 12/07
Methods: [MiniPrep]
Protocol: Fermentas protocol
Notes: pellet from 10 mL ON-culture was resuspended in 500uL resuspension buffer, and transferred into two eppendorf tubes, which were run in parellel
samples were loaded into a 1% gel with a gene ruler red marker.
Results:
Transformation of MG1655 e. coli with BBa_274210
Start Date: 13/07 End Date:
Methods: ON cultures, Competent Cells, Transformation of competent cells.
Protocols:Competent Cells, transformation
Experiment: Making Competent e. coli MG1655
Date: 13/07
*ON cultures of strain MG1655 were put over the night before.
Eksponential growth was started at 09.30 with an OD of .19A.
 "
"
