Team:SDU-Denmark/labnotes
From 2010.igem.org
(Difference between revisions)
(→Colony PCR of K274210 in pSB1A2 (transformation from 08/07)) |
(→Group: Flagella) |
||
| Line 22: | Line 22: | ||
--[[User:Louch07|Louch07]] 15:00, 9 July 2010 (UTC) | --[[User:Louch07|Louch07]] 15:00, 9 July 2010 (UTC) | ||
<br><br> | <br><br> | ||
| + | |||
| + | |||
| + | === Polyferation of FlhDC, FlhD and FlhC genes === | ||
| + | |||
| + | ''Methods:'' PCR and Gel electrophoresis | ||
| + | |||
| + | ''Notes:'' Since our FldhDC primers have yet to work, we have decided to test them on previously purified cromosomal DNA. Examination of the primers showed that the FlhC reverse primer had a melting temperature of only 45˚C. Therefore we decided to run the samples on a gradient PCR. Simultaneously, we prepared 2 extra samples, isolating FlhD and FlhC, respectively. We did this because we wanted to see if our problems were caused because the combined gene-sequence was to long (932bp). | ||
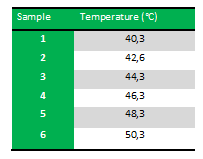
| + | Because we just wanted to test our primers in this PCR, we used Taq polymerase, because although it doesn’t proofread, it is remarkably cheaper than Pfu. On the [http://www.fermentas.com/en/products/all/pcr-qpcr-rt-pcr/standard-pcr/ep028-taq-dna-native Fermentas homepage] we found that the annealing temperature for Taq is Tm-5 , which in this case means 40˚C. However, Taq polymerase is not very effective at temperatures under 50˚C so we designed the gradient to lies between 40 and 55˚C. More specifically we chose the following temperatures: | ||
| + | |||
| + | [[Image:Team-SDU-Denmark-PCR_temp_for_FlhDC.png]] | ||
| + | |||
| + | ''Results:'' The experiment was succesfull! We could detect FlhDC DNA in the PCR's run between 42.6˚C and 48.3˚C. FlhD DNA in PCR’s run between 40.3˚C and 44.3˚C and also between 48.3˚C and 50.3˚C. FlhC DNA in PCR’s run between 40.3˚C and 50.3˚C. | ||
== Group: Photosensor == | == Group: Photosensor == | ||
Revision as of 08:10, 12 July 2010
 "
"