Team:Heidelberg/Project/miMeasure
From 2010.igem.org
(→Results) |
(→Results) |
||
| Line 23: | Line 23: | ||
==Results== | ==Results== | ||
| + | |||
| + | |||
===Analysis of Randomized Binding Sites Against Synthetic miRNA=== | ===Analysis of Randomized Binding Sites Against Synthetic miRNA=== | ||
Revision as of 19:41, 27 October 2010

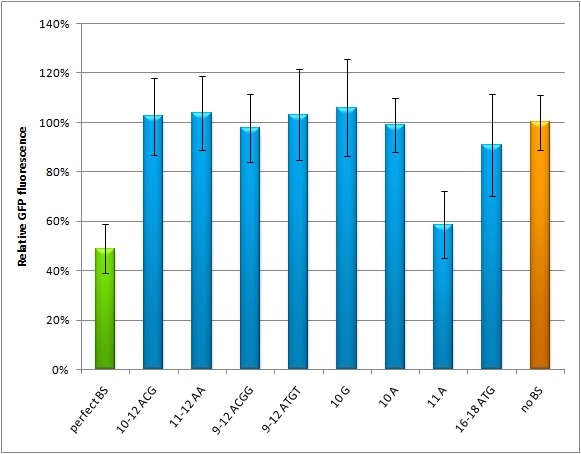
miMeasureAbstractWith the rising importance of small RNA molecules in gene therapy the identification and characterization of miRNAs and their binding sites become crucial for innovative applications. In order to exploit the miRNA ability to target and regulate specific genes, we constructed a measurement standard not only to characterize existing miRNAs but also to validate potential synthetic miRNAs for a new therapeutic approach. The synthetic miRNAs we created are inert for endogenous targets and thus applicable for gene regulation without any direct side effects. This opens new possibilities for precise expression tuning, especially in quantitative means. Our miMeasure construct plasmid (see sidebar) normalizes knockdown of the green fluorescent protein (EGFP) to the blue fluorescent protein (EBFP2). This allows for an accurate study of binding site properties, since both fluorescent proteins are combined in the same construct and driven by the same [http://partsregistry.org/Part:BBa_K337017 bidirectional promoter]. Another advantage is, that any desired binding site can be cloned easily into the miMeasure plasmid with the [http://dspace.mit.edu/handle/1721.1/45139 BB-2 standard]. As the binding site is inserted downstream of EGFP, a regulation of EGFP expression is to be expected. The percentage of knockdown of each modified binding site can be calculated by the ratio of EGFP to EBFP2. This ratio is derived from a linear regression curve. Therefore the knock-down efficiency can be determined by various basic methods e.g. plate reading, flow cytometry or microscopy. IntroductionMicro RNAs regulate mainly the translation of their target genes by preferably interacting with regions in the 3’ untranslated region (UTR) of their target mRNA. Base-pairing with the miRNA binding site (BS) causes formation of diverse miRNA-mRNA duplexes [http://2010.igem.org/Team:Heidelberg/Project/miMeasure#References (reviewed by Fabian et al., 2010)]. The BS consists of a seven nucleotide long seed region that is perfectly matched to the miRNA, and surrounding regions that matched partially. The seed region is defined as being the minimal required base-pairing that can regulate the mRNA. Apart from the seed region, binding can be unspecific, creating mismatches and bulges. The position and properties of the bulges seem to play a central role in miRNA binding and therefore knockdown efficiency [http://2010.igem.org/Team:Heidelberg/Project/miMeasure#References (reviewed by Bartel et al., 2009)]. Since we were going to use synthetic miRNA BS in our genetherapeutic approach, we had to find a way to study their effects in a standardized manner that would be comparable and reproducible. One goal of the iGEM Team Heidelberg 2010 was to test the effects of changes in BS sequences on expression control. Thereby miRNA BS should be characterized. To standardize our measurements of knockdown according to BS specificity, we had to come up with a new standard that is independent from the endogenous cell machinery. We decided to bring synthetic miRNAs into play, hence we engineered BS for them creating an artificial regulatory circuit. Of course there are also differences that arise through the availability of the enzymes involved in the miRNA pathway that may differ slightly from cell to cell. Therefore, we also measured the knockdown achieved by the perfect binding site and set this as 100% knockdown efficiency. Ideally, the miRNA would be stably expressed in the cell line, but a uniform co-transfection also leads to an even distribution of synthetic shRNA-like miRNAs (shRNA miRs). Additionally, miRNA levels can be adjusted by differing transfection ratios. The main purpose of our measurement standard, miMeasure, is to express two nearly identical but discernible proteins: one of them tagged with a BS, the other one unregulated (even though the possibility exists to clone in a reference binding site). The two reporters are expressed by a bidirectional CMV promoter to make sure their transcription rate is comparable. We used a destabilized version of GFP, dsEGFP and a dsEBFP2 that was derived from the same sequence (Ai et al., 2007). Thus, we could make sure that both proteins exhibit the same synthesis and degradation properties, making them directly comparable. Hereby, we can also neglect the difference between mRNA and protein knockdown and can take the fluorescence of the marker protein as a direct, linear output of mRNA down-regulation. We included a BBb standard site into our plasmid, which allows to clone BS behind the GFP. If co-transfected with the corresponding shRNA miR, GFP will be down-regulated, while BFP expression is maintained. The ratio of GFP to BFP expression can be used to conclude the knockdown efficiency (in percent, compared to perfect binding site=100% and no binding site=0%) of the BS. Having destabilized marker proteins with a turnover time of two hours enables us not only to avoid accumulation of marker proteins, which would make the knockdown harder to observe, but also to conduct time-lapse experiments. In the future, this could be for example a way to observe dynamic activity patterns of endogenous miRNAs. ResultsAnalysis of Randomized Binding Sites Against Synthetic miRNAConfocal microscopy measurementsWe used microscopy analysis to determine the EGFP expression in relation to EBFP2. EBFP2 serves as a normalization for transfection efficiency. Nine miMeasure constructs with different binding sites were designed. Those were cloned downstream of EGFP behind the miMeasure construct, whereas the EBFP2 expression stays unaffected. The GFP/BFP-ratio stand for the level of GFP-expression normalized to one copy per cell. We modified binding sites for the synthetic miRNA by introducing random basepairs surrounding the seed region as described above, thereby changing the knockdown efficiency. In figure 1 we plotted the knockdown percentage of our constructs. The miMeasure construct and negative control were co-transfected with either shRNA miRsAg expressed from a CMV promoter on pcDNA5 or an inert synthetic RNA as control in 1:4 ratio, respectively.
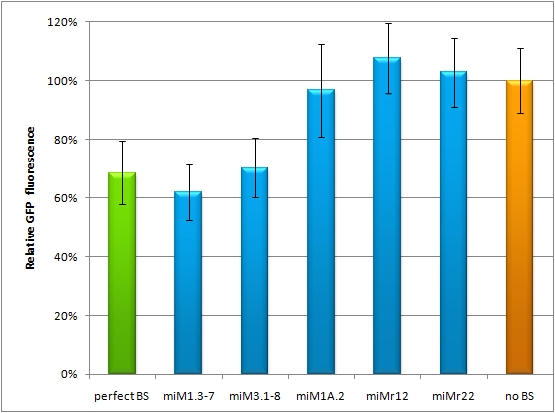
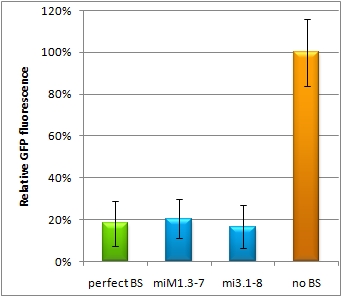
Flow cytometry measurementsHela cells transfected with the constructs (described above) are also taken for flow cytometry. 10000 cells are measured. The cells are plotted on a logarithmic scale in relation to EGFP and EBFP2 intensity. Each dot represents one fluorescent cell. The two different coloured dots belong to two different measurements. Both sets of dots make up a line on the logarithmic, which shows the correlation of EGFP and EBFP2 very well. The orange dots represent cells, which were transfected with different miMeasure constructs and the miRsAg. The blue dots represent cells, which were also transfected with the different miMeasure constructs, but here the miR-155 is cotransfected, which doesn't have any specificity for the binding sites. The cotransfection with miR-155 is therefore the negative control. If the dots become white, it means the orange and blue ones overlap. So both lines overlap in the negative control, whereas the orange line is going up for the miMeasure construct with the perfect binding site. All the other constructs look like the negative control. The line is more scattered on the linear scale, although the shifting of the orange dots are more visible for the construct containing the perfect binding site. All the other constructs have the same range of scattering as the negative control. 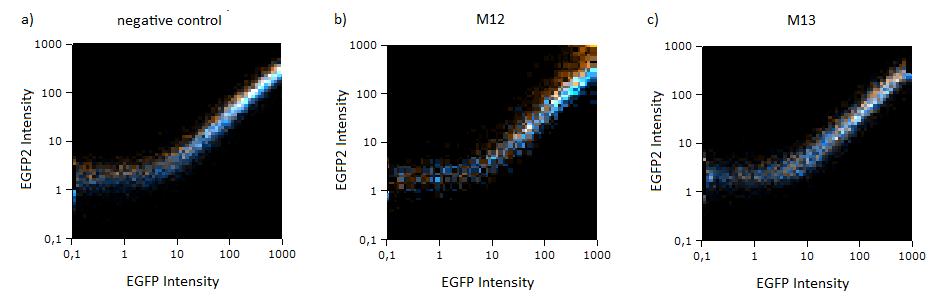 GFP/BFP correlation of single transfected Hela cells according to flow cytometry analysis on a logarithmic scale different binding sites for miRsAg cotransfected with miRsAg or with mi-R155, respectively. The orange dots represent the cotransfected cells with miRsAg and the blue dots the cotransfected cells with miR-155. Hela cells were used. 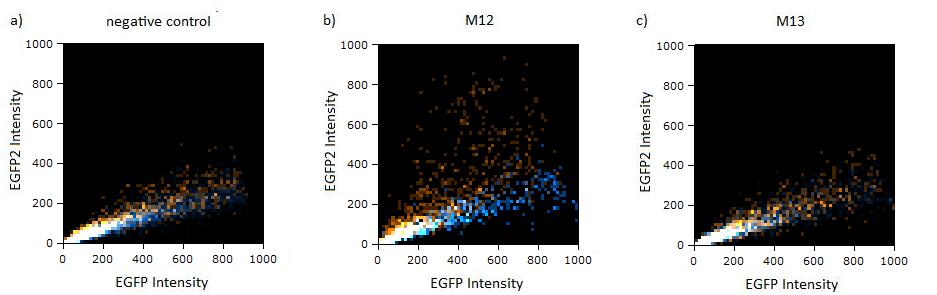 GFP/BFP correlation of single transfected Hela cells according to flow cytometry analysis on a linear scale different binding sites for miRsAg cotransfected with miRsAg or with mi-R155, respectively. The orange dots represent the cotransfected cells with miRsAg and the blue dots the cotransfected cells with miR-155. Hela cells were used. Analysis of miRaPCR Generated Binding Sites Against a Natural miRNAThe miRaPCR generates binding sites out of rationally designed fragments. These are aligned with each other by chance, whereby different spacer regions are inserted randomly in between. It has been suggested that having more than one binding site of for the same miRNA behind each other can lead to stronger down-regulation than a single one. If imperfect binding sites are aligned, it is also supposed to be stronger than a single one. This is what we tested using MiRaPCR for effortless assembly of binding site fragments. For our experiments, we took advantage of the high abundance of miRNA 122 in liver cells and tested different combinations of binding sites created by miRaPCR. We transfected HeLa and HuH7 cells with the constructs described in table 2. Since HuH7 cells express miR-122, the constructs will be affected in the HuH7 cells without cotransfecting any miRNAs, whereas miR-122 were cotransfected for the HeLa cells in 2:1 ratio. The result shows the ratio between GFP and BFP normalized to the negative control (miMeasure constructs without binding sites). The miMeasure constructs were also compared to the expression of miMeasure containing one perfect binding site for miRNA 122. The Hela cells transfected with the different constructs are also imaged with the epifluorescent microscope. The cells in the negative control (miMeasure with perfect binding site cotransfected with miR-155, see a)are mostly green, whereas the cells with the miMeasure construct containing the perfect binding sites (see b) are mostly blue.  epifluorescent microscopy image (10x) of Hela cells transfected with miMeasure miMeasure with a perfect binding site is a) cotransfected with miR-155, which has no specificity to miR-122, b) cotransfected with miR-122, which is complementary to the perfect binding site. EGFP is regulated by miR-122, EBFP2 is unregulated and serves as transfection control.
The Huh7 cells were also transfected with the 4 different constructs. Here a cotransfection with miR-122 is not necessary, since Huh7 cells express miR-122 themselves. The knock-down of the perfect binding sites are stronger than the knock-down in the Hela cells. Here the knock-down efficiency is 80% for the perfect binding site and the aligned constructs.
Flow cytometryThe same constructs in Hela cells were analyzed by flow cytometry, too. Here the orange dots also represent the miMeasure construct transfected with the specific miRNA and the blue dots make up the negative control.
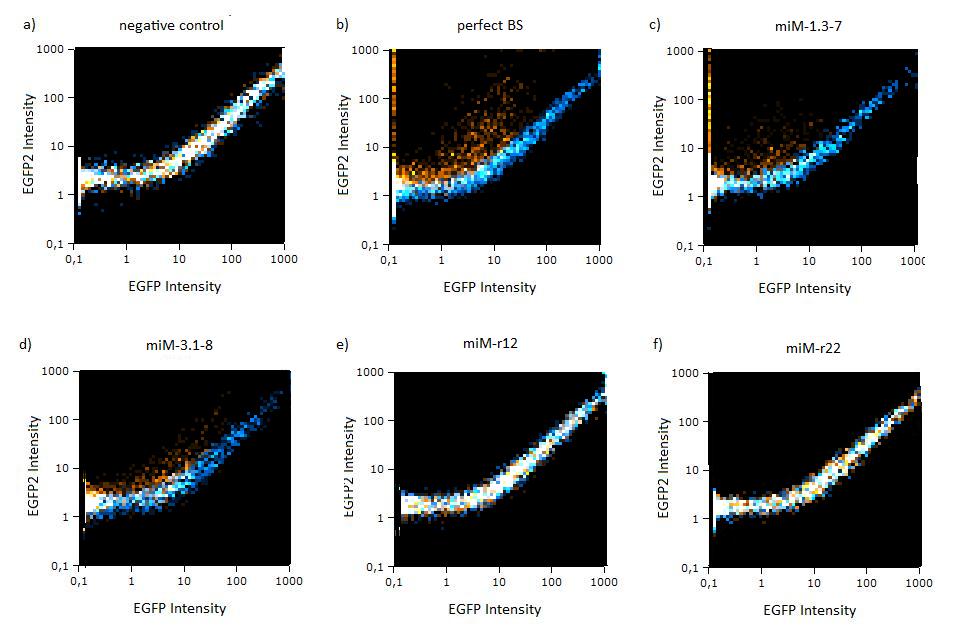 GFP/BFP correlation of single transfected Hela cells according to flow cytometry analysis different binding sites for miR122 cotransfected with miR-122 or with miR-155, respectively. The orange dots represent the cotransfected cells with miR122 and the blue dots the cotransfected cells with miR-155. Hela cells were used.
Analysis of endogenous miRNADiscussionMethodsThe fluorescence of GFP and BFP can be compared using different methods, for example automated fluorescence plate reader systems, flow cytometry or manual and automated fluorescence microscopy. ReferencesAi HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry. 2007 May 22;46(20):5904-10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009 Jan 23;136(2):215-33. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351-79.
|
||||||||||||||||||||||||||||||||||||||||
 "
"