Team:St Andrews/project/modelling/model 3
From 2010.igem.org
(→HSL production & degradation) |
(→LuxR degradation) |
||
| Line 106: | Line 106: | ||
[[Image:LuxR Degradation Initial Test.jpg|center|800px]] | [[Image:LuxR Degradation Initial Test.jpg|center|800px]] | ||
| - | + | '''Figure 9: Parameter sensitivity test results on LuxR degradation rate''' | |
| - | '''Figure 9''' | + | |
Revision as of 19:04, 27 October 2010


Model 3: Pseudo Multi Cell One Dimension
Contents |
Theory & assumptions
The results produced from both of our previous models were unsatisfactory, since neither gave us quantifiable data from which experimental comparisons could be made, although the switching on was clearly visible in both. Therefore it was decided that in order to properly capture the mechanism of bistability it was essential to have some element of cell number, and as such we must simulate the growth of a cell colony rather than artificially increase the amount of HSL in our external environment. Several crucial elements were added to the model to incorporate this new outlook:
i. A new variable 'Number of cells' was added
ii. Two new functions which simulate cell growth and cell death
iii. A new paramter 'Volume'
The number of cells is set at the beginning of the simulation by the user. Based on a doubling time of 20 minutes, the variable is increased every increment by the cell growth function. In order to simulate the increase in HSL due to these new cells, each new cell is accompanied by an increase in HSL which is equal to that being contributed by the cells already present in the system. (VOLUME THING IN DIFFERENTIALS). Similarly we included a cell death function which decreases the number of cells at a halving rate of 20 minutes in tandem to the growth rate.
Results
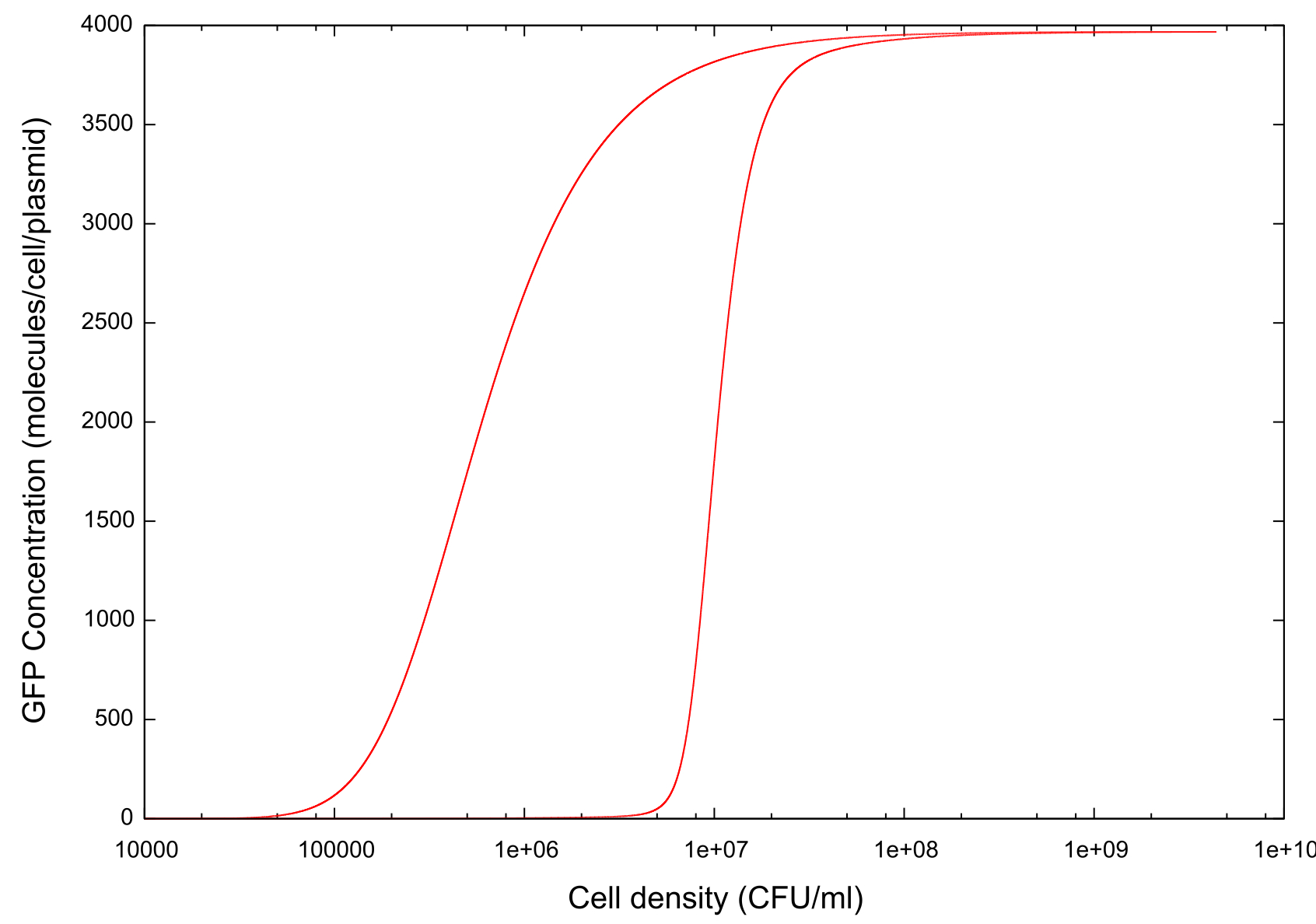
On running the model through the cell growth, stationary and cell death phases, the first example of ‘true bistability’ is produced, see figure . Since this model contains spatial dimensions we are able to determine a specific cell density at which the switch happens, and this is comparable to the value predicted by other models and by experimental evidence. There is also a clear difference between the switch-on and switch-off thresholds which we hypothesise is caused by…
Figure 1: Graph showing change in GFP concentration against cell density
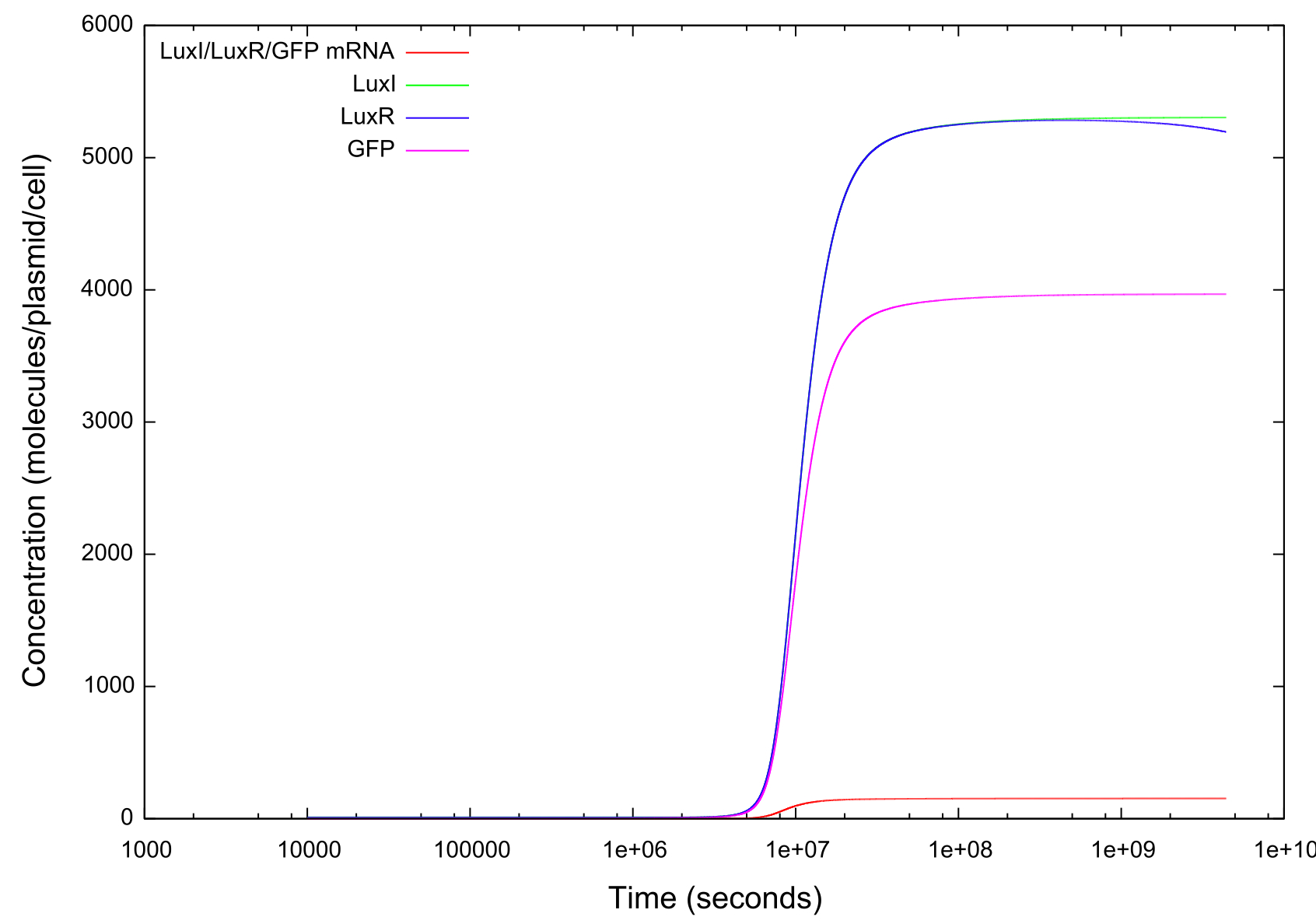
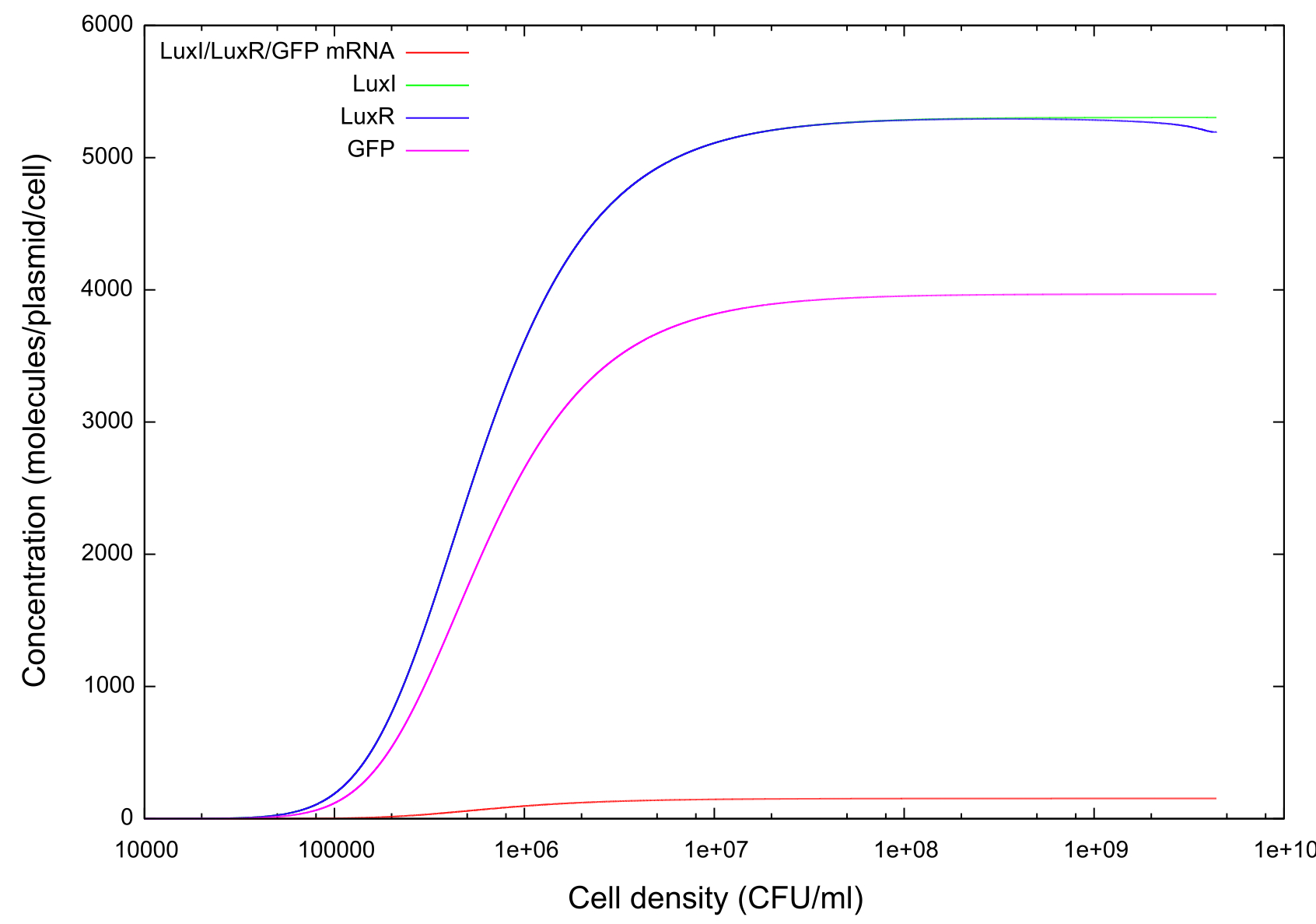
Figure 2: Graph showing change in GFP, LuxR, LuxI and mRNA concentrations for the transition from off to on
Figure 3: Graph showing change in GFP, LuxR, LuxI and mRNA concentrations for the transition from on to off
Info on HSL-LuxR concentration behaviour
Graph with up and down data?
Parameter Testing
The next stage in the development of our model was to probe which factors had an impact on the behaviour of the system and exactly what that impact was and what implications they have.
Measuring bistability
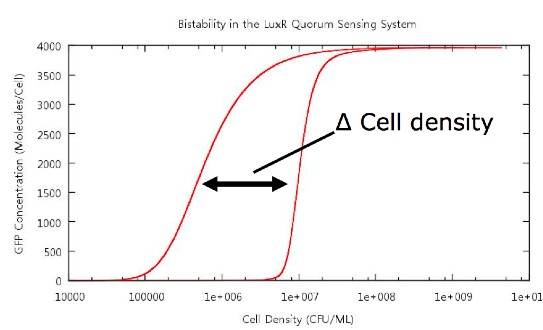
The key point which we wished to investigate was the bistability of our system, so in order to do this we had to develop a standard way in which we would measure the 'level' of bistability. The method decided upon was to take a reading of the cell density at a fixed value of GFP concentration during cell growth and similarly record the cell density at the same GFP concentration during cell death. Thus we would obtain a value Δ Cell density which gives us a useful quantity which we can use in our tests.
Figure 4: Our method of bistability measurement
Testing our switch
We decided that there were some key parameters which we would like to test, which were the rates of:
- Association and dissociation
- HSL production and degradation
- LuxR degradation
- Promoter binding site strengths
Implementation
In order to test the level of bistability, we first needed to find the parameter range for which the system switched on. This was done by running our model many times with different values of the parameter we wished to investigate, and looking at for which values the system switched on by the final concentration of GFP. We could then operate only within the operating range of the switch and run our model many times within this range, looking at the value of ΔCell density and comparing for different parameter values.
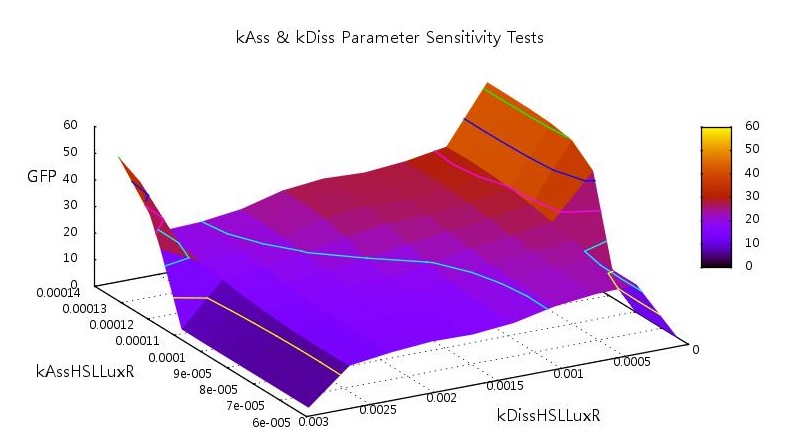
Association and dissociation of HSL-LuxR
The HSL-LuxR is the key to the entire quorum sensing circuit and as such should play an important role in the level of bistability of the system. Our initial tests showed unsurprising results, that at high dissociation rates the system requires a higher rate of association to switch on and vice-versa.
Figure 5: Parameter sensitivity tests on rate of associations and dissociation on HSL and LuxR
HSL production & degradation
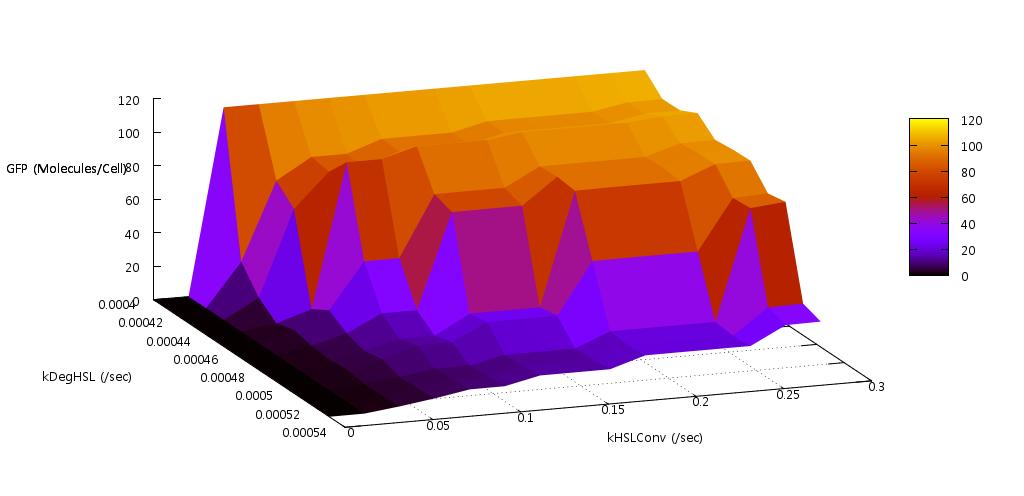
We reasoned that the rate at which HSL was both produced, and degraded within the system should have a significant effect on the bistability since this molecule is fundamental to the process of quorum sensing in E.coli. Indeed our initial tests found that if the conversion rate was too low, or the degradation rate too high, the system did not actually switch on (see figure ).
Figure 6: Results from initial parameter tests on rate of HSL production and degradation
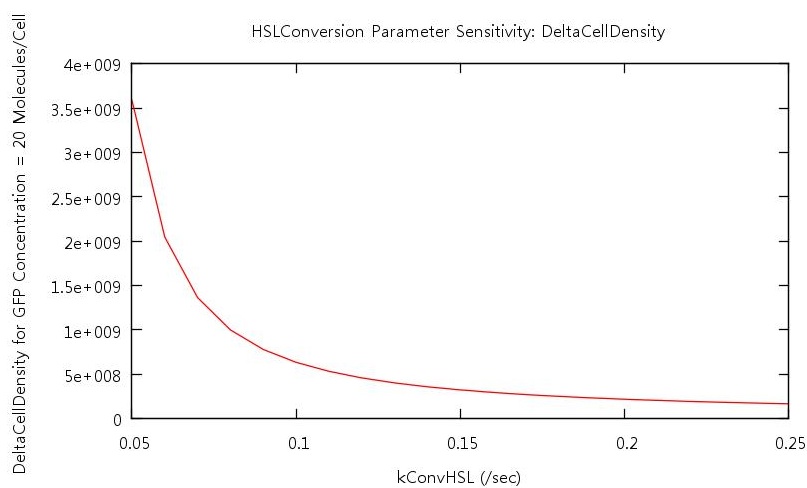
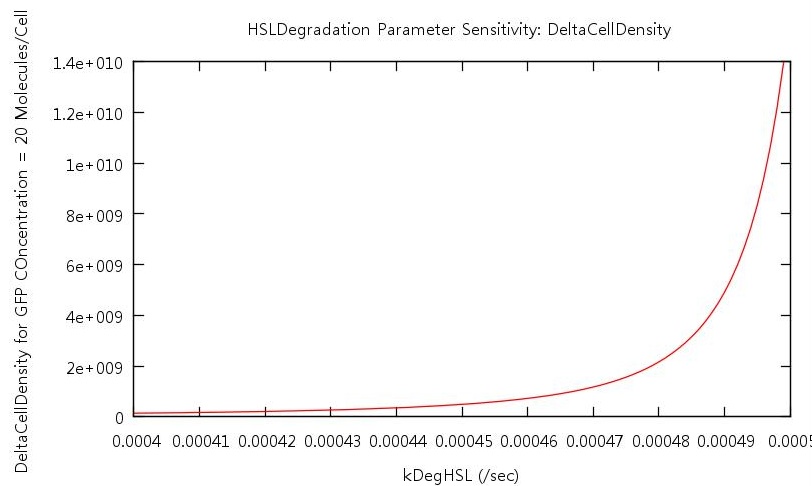
Our test indicated that our parameter test should be performed for values, 0.05 < kConvHSL < 0.25 and 0.0004 < kDegHSL < 0.0005. The results of these tests are shown below.
Figure 7: Parameter sensitivity test on rate of HSL conversion
Figure 8: Parameter sensitivity test on rate of HSL degradation
From these plots we can clearly see that by increasing the HSL conversion rate we also decrease the bistability of the system and by increasing the HSL degradation rate we increase the bistability of the system.
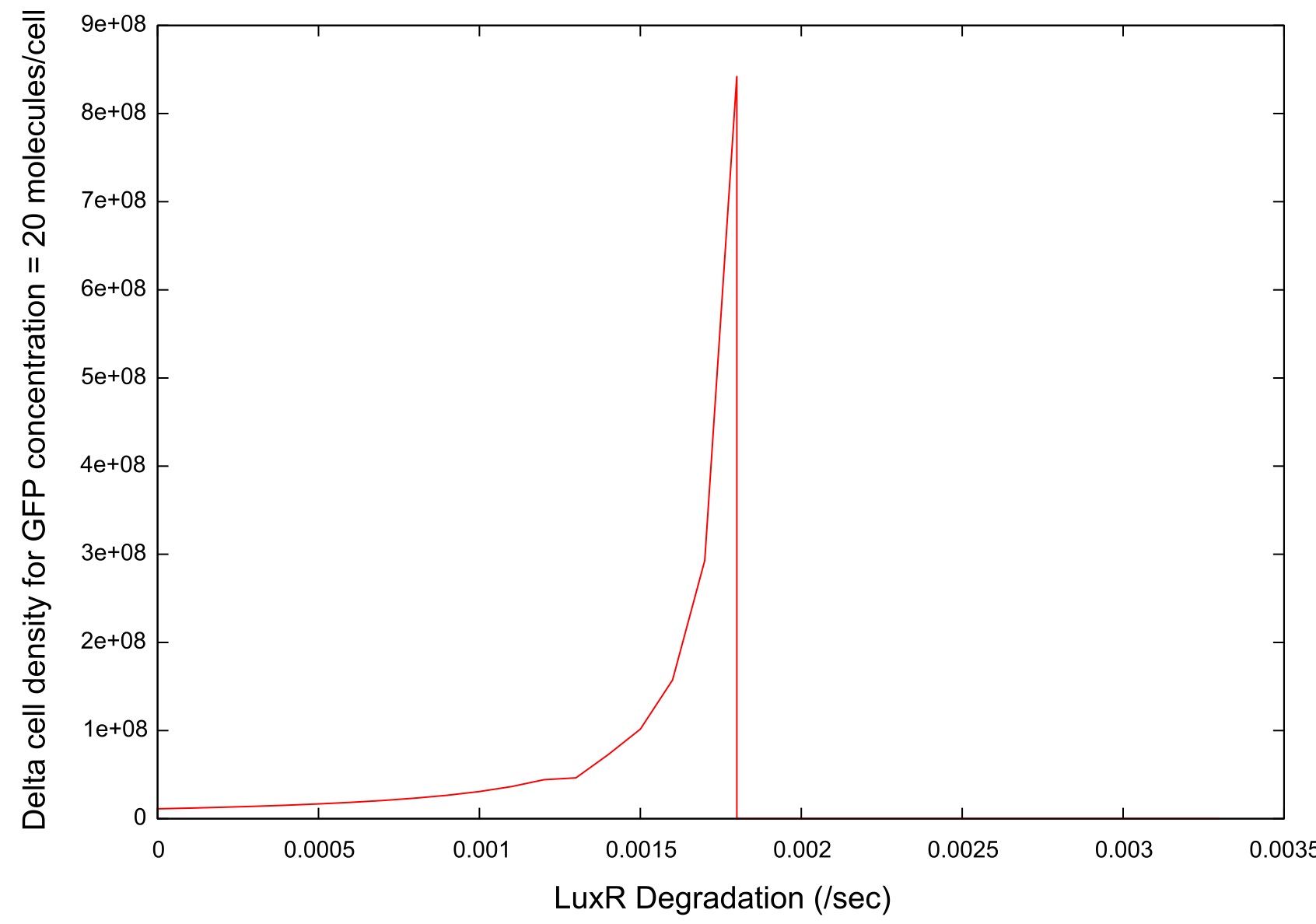
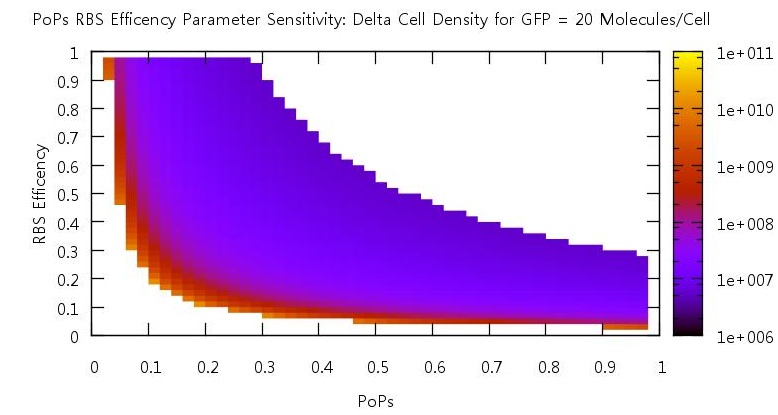
LuxR degradation
Similar tests were performed on the effects of changing the rate of LuxR degradation. We also expected interesting results from these tests since the LuxR is the HSL binding molecule. Our initial test to find a suitable parameter range gave us the data shown in figure .
Figure 9: Parameter sensitivity test results on LuxR degradation rate
There is a clear drop to zero in the bistability at a certain threshold level for LuxR degradation. This is due to the fact that the LuxR is degrading before an can bind to the HSL so the system does not switch on.
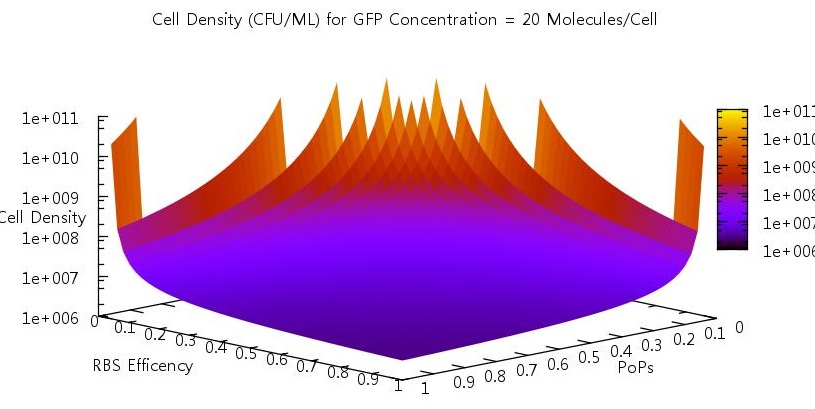
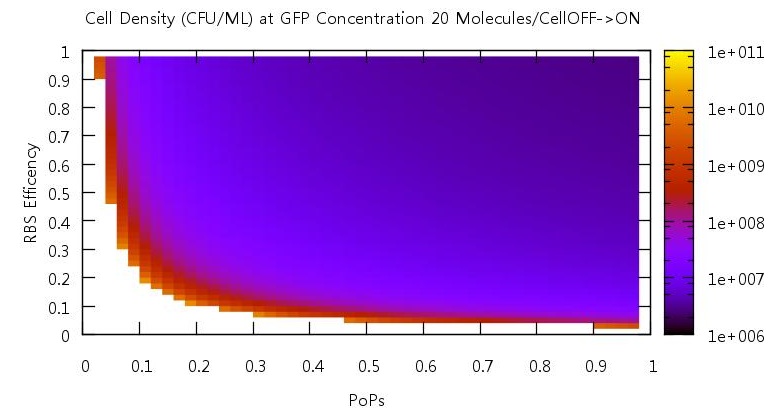
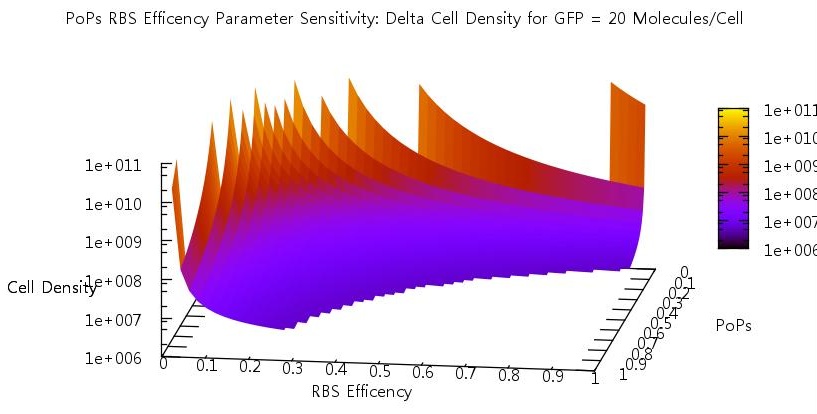
Promoter efficiency and affinity
There are two aspects of the quorum sensing network over which we as experimental scientists can control: the speed at which ribosomes translate our proteins and the binding of the promoter. In our model, these two phenomena correspond to the rate of translation kTranslation and kMaxProductionRNA. Thus we felt it would be very useful if we could characterise the beahvaiour of our switch on changing these parameters, since if the switch is to be put to any practical use it will be these parameters which would experimentally be changed. Thus we performed parameter tests changing these rates simultaneously to investigate the effects.
Figure 10
Figure 11
Figure 12
Figure 13
 "
"