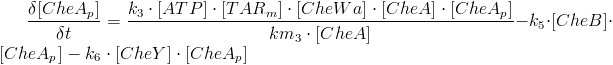Team:SDU-Denmark/project-bc
From 2010.igem.org
(Difference between revisions)
m (→Model results) |
(→The real system) |
||
| Line 13: | Line 13: | ||
=== The real system === | === The real system === | ||
| - | Several chemosensory systems exist in bacteria. The one most studied is the chemotaxis of ''E. coli''. Polar clusters of membrane-spanning methyl accepting chemotaxis proteins (MCPs) are positioned in the ends of the rod shaped ''E. coli''.<br> | + | Several chemosensory systems exist in bacteria. The one most studied is the chemotaxis of ''E. coli''. Polar clusters of membrane-spanning methyl accepting chemotaxis proteins (MCPs) are positioned in the ends of the rod shaped ''E. coli''[[https://2010.igem.org/Team:SDU-Denmark/project-bc#References 1]].<br> |
Tar (responding to aspartate), Tsr (responding to serine), Tap (responding todipetides), Trg (responding to galactose) ang Aer (responding to oxygen) are the most common of these receptors seen in ''E. coli'' strains[[https://2010.igem.org/Team:SDU-Denmark/project-bc#References 1]]. Transduction of the signal between the MCPs and the flagella motor is managed by a particular fine-tuned signalling cascade, which is governed by several intracellular proteins.<br> | Tar (responding to aspartate), Tsr (responding to serine), Tap (responding todipetides), Trg (responding to galactose) ang Aer (responding to oxygen) are the most common of these receptors seen in ''E. coli'' strains[[https://2010.igem.org/Team:SDU-Denmark/project-bc#References 1]]. Transduction of the signal between the MCPs and the flagella motor is managed by a particular fine-tuned signalling cascade, which is governed by several intracellular proteins.<br> | ||
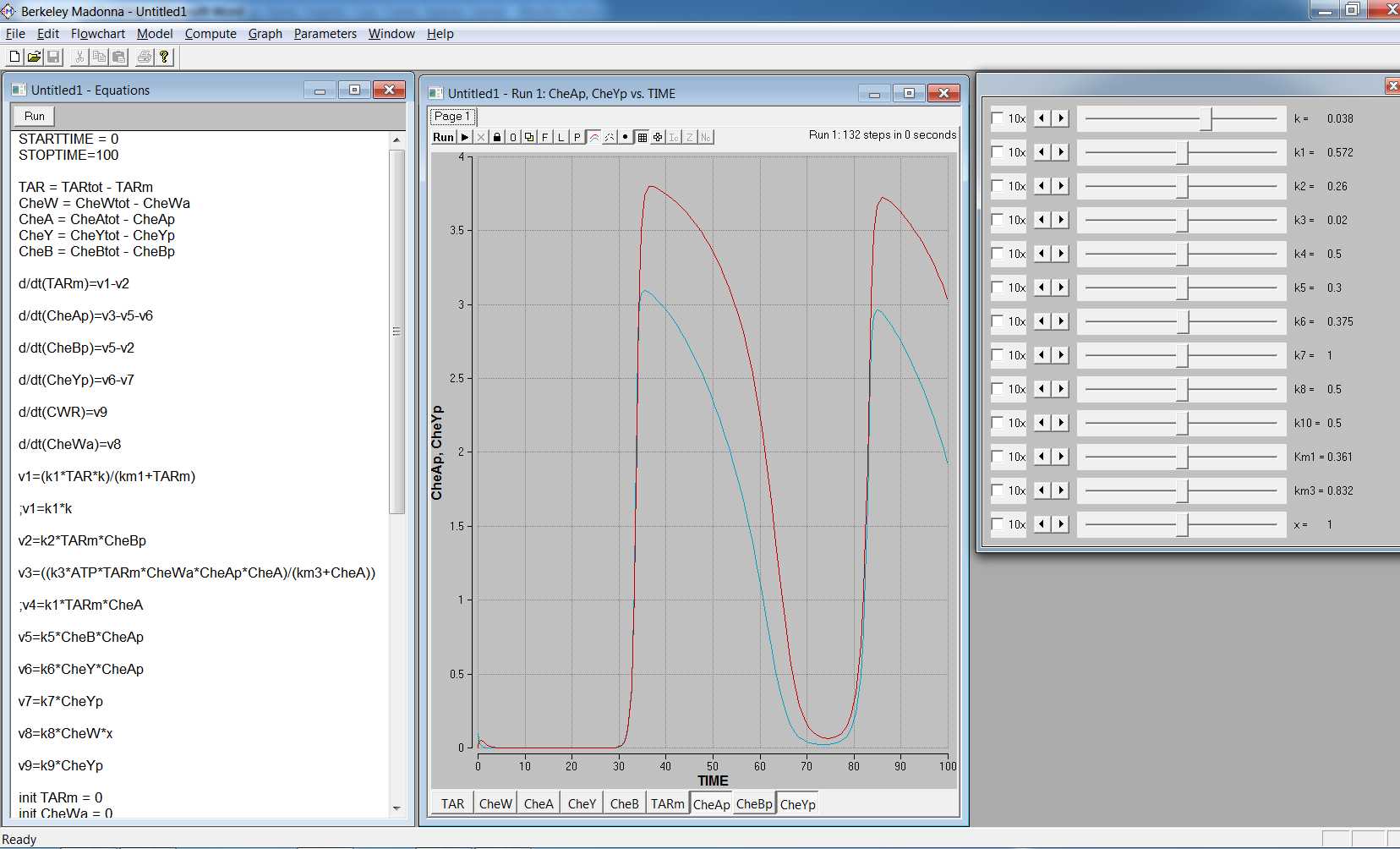
[[Image: Team-SDU-Denmark-Chemotaxis.png |thumb|center|550px| '''Figure 1:''' Schematics of the bacterial chemotaxis pathways [[https://2010.igem.org/Team:SDU-Denmark/project-bc#References 1]]]] | [[Image: Team-SDU-Denmark-Chemotaxis.png |thumb|center|550px| '''Figure 1:''' Schematics of the bacterial chemotaxis pathways [[https://2010.igem.org/Team:SDU-Denmark/project-bc#References 1]]]] | ||
Chemotaxis consists of three important phases: reaction, adaptation and relaxation. In the reaction phase, one of the MCPs sense the specific chemical gradient it responds to and the membrane protein activates the CheW enzyme. The active CheW enzyme suppresses the auto-phosphorylation of the CheA enzyme and when no or very little CheA enzyme is phosphorylated, no phosphorylation of the CheY and CheB enzymes occurs. In the end, this increases run, because phosphorylated CheY binds to the flagella motors and induce tumbling. When the bacteria have reached the area with a higher concentration of the chemical in question adaptation sets in.<br><br> | Chemotaxis consists of three important phases: reaction, adaptation and relaxation. In the reaction phase, one of the MCPs sense the specific chemical gradient it responds to and the membrane protein activates the CheW enzyme. The active CheW enzyme suppresses the auto-phosphorylation of the CheA enzyme and when no or very little CheA enzyme is phosphorylated, no phosphorylation of the CheY and CheB enzymes occurs. In the end, this increases run, because phosphorylated CheY binds to the flagella motors and induce tumbling. When the bacteria have reached the area with a higher concentration of the chemical in question adaptation sets in.<br><br> | ||
Because no CheB have been phosphorylated during the reaction period CheR is “allowed” to methylate the MCPs, which increases the auto-phosphorylation of the CheA enzyme. This increase in auto-phosphorylation of CheA results in phosphorylation of CheY that leads to tumbling of the bacteria. In the last phase, relaxation, CheB has again been phosphorylated by CheA and is now able to demethylate the MCPs, returning the bacteria to its normal run/tumble frequency with a 0,1 second tumbling period every 1 second.<br><br> | Because no CheB have been phosphorylated during the reaction period CheR is “allowed” to methylate the MCPs, which increases the auto-phosphorylation of the CheA enzyme. This increase in auto-phosphorylation of CheA results in phosphorylation of CheY that leads to tumbling of the bacteria. In the last phase, relaxation, CheB has again been phosphorylated by CheA and is now able to demethylate the MCPs, returning the bacteria to its normal run/tumble frequency with a 0,1 second tumbling period every 1 second.<br><br> | ||
| - | One other thing to consider in the model is the distance from the CheY phosphorylation site to the flagella motors that are randomly located on the entire cell body. Several modelling methods have been proposed and nearly all of them include these considerations: <br> | + | One other thing to consider in the model is the distance from the CheY phosphorylation site to the flagella motors that are randomly located on the entire cell body[[https://2010.igem.org/Team:SDU-Denmark/project-bc#References 1]]. Several modelling methods have been proposed and nearly all of them include these considerations[[https://2010.igem.org/Team:SDU-Denmark/project-bc#References 1]]:<br> |
"Attractant is assumed to be in excess and binding is assumed to be rapid". One of the most common receptor types to be modelled is the Tar receptor. This is not unexpected given that a large degree of the experimental literature has focused on the response of ''E. coli'' to aspartate which is detected by this receptor. Tar is also one of the most abundant receptors within the cytoplasmic membrane.<br> | "Attractant is assumed to be in excess and binding is assumed to be rapid". One of the most common receptor types to be modelled is the Tar receptor. This is not unexpected given that a large degree of the experimental literature has focused on the response of ''E. coli'' to aspartate which is detected by this receptor. Tar is also one of the most abundant receptors within the cytoplasmic membrane.<br> | ||
In models which have focused on the adaptation and/or the phosphorylation cascade, receptors are commonly modelled as complexes consisting of MCP, CheW and CheA. This is justified by the tight association of CheW and CheA to the MCP receptors.<br> | In models which have focused on the adaptation and/or the phosphorylation cascade, receptors are commonly modelled as complexes consisting of MCP, CheW and CheA. This is justified by the tight association of CheW and CheA to the MCP receptors.<br> | ||
Revision as of 16:00, 27 October 2010
 "
"