Team:Newcastle/6 September 2010
From 2010.igem.org
Swoodhouse (Talk | contribs) (→Sucrose/Levan's glue) |
Swoodhouse (Talk | contribs) (→Sucrose/Levan's glue) |
||
| Line 3: | Line 3: | ||
=Sucrose/Levan's glue= | =Sucrose/Levan's glue= | ||
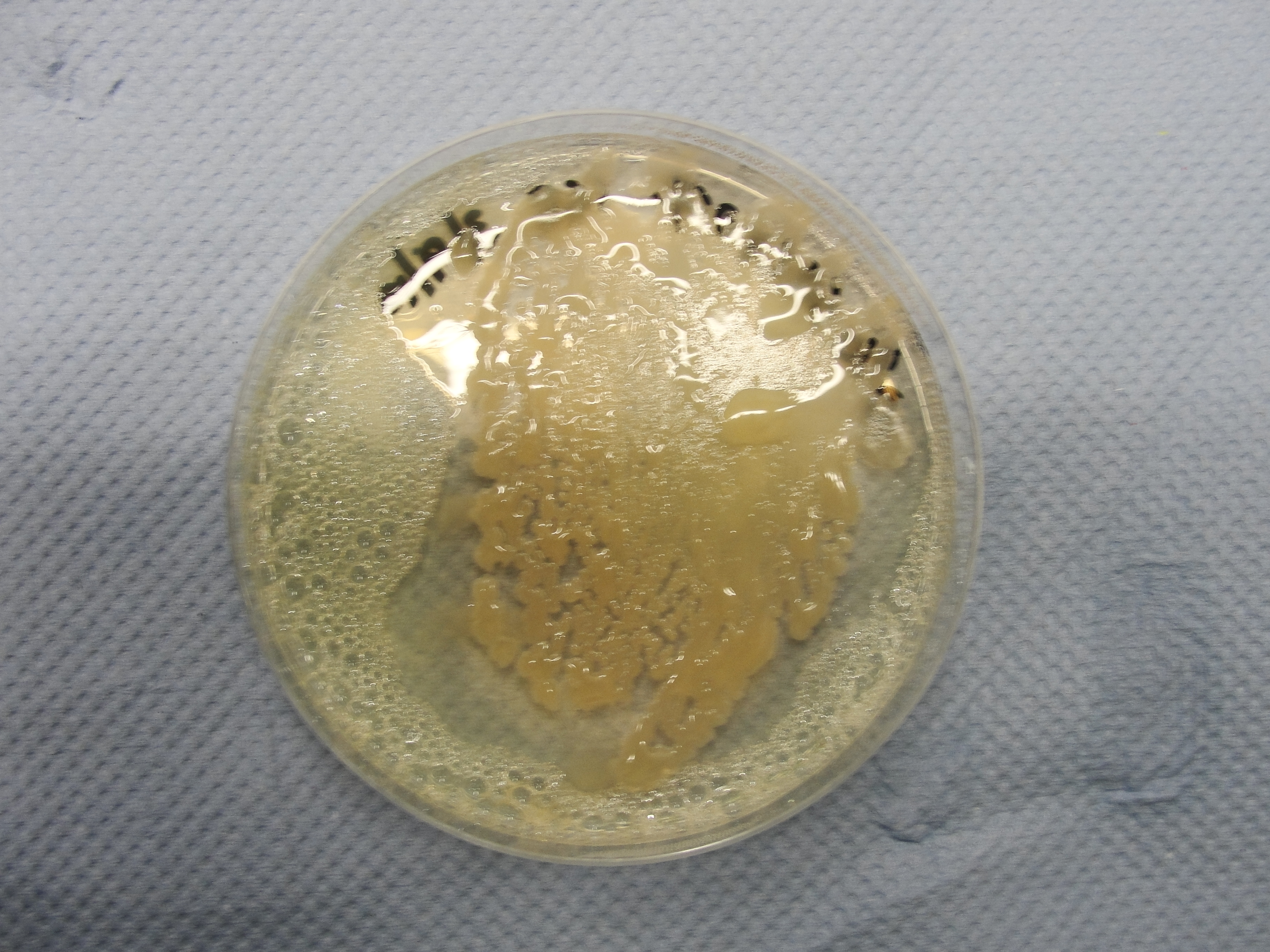
| - | We started working on | + | We started working on levans. For this we used agar plate containing 10% sucrose and ''Bacillus subtilis'' 168 was grown onto that medium. The sucrose cultures and controls were compared. The cultures grown on sucrose should be considerably thicker. |
The overnight cultures of the different strains were also grown on solid agar plates with and without sucrose. | The overnight cultures of the different strains were also grown on solid agar plates with and without sucrose. | ||
| - | For more information | + | For more information levan, please refer to: [[Team:Newcastle/glue|levan]]. |
===PHOTOS=== | ===PHOTOS=== | ||
| - | [[Image:DSC01022.JPG|500px|''B. subtilis producing | + | [[Image:DSC01022.JPG|500px|''B. subtilis producing levan]] |
| - | '''Image 1''': Image showing ''B. subtilis'' producing | + | '''Image 1''': Image showing ''B. subtilis'' producing levan on agar plate containing 10% sucrose. |
=Hyperspankoid characterisation= | =Hyperspankoid characterisation= | ||
Revision as of 14:25, 27 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Sucrose/Levan's glue
We started working on levans. For this we used agar plate containing 10% sucrose and Bacillus subtilis 168 was grown onto that medium. The sucrose cultures and controls were compared. The cultures grown on sucrose should be considerably thicker. The overnight cultures of the different strains were also grown on solid agar plates with and without sucrose. For more information levan, please refer to: levan.
PHOTOS
Image 1: Image showing B. subtilis producing levan on agar plate containing 10% sucrose.
Hyperspankoid characterisation
Aims
The aim of these experiments is to ligate the promoters hyperspankoid, pspacoid and hyperspank in front of rfp on the pSB1C3 plasmid seperately. In order to do this, the first step is to amplify the three promoter sequences and the pSB1C3 plasmid using Phusion PCR.
Materials and Protocol
Please refer to Phusion PCR for Phusion PCR protocol.
The details of the four PCR reactions are included in the table below:
| Tube | Part to be amplified | DNA fragment consisting the part i.e. template | Forward primer | Reverse Primer | Melting Temperature (Tm in °C) | Size of the fragment (in bp) | Extension time (in seconds) |
|---|---|---|---|---|---|---|---|
| 1 | Hyperspankoid | yneA | Prom_for | HpSpanPspacoid_rev | 65 | 150 | 15 |
| 2 | Pspacoid | kinA | Prom_for | HpSpanPspacoid_rev | 65 | 150 | 15 |
| 3 | Hyperspank | K143055 from Bacillus plate - BS022 | Prom_for | Pspac-hy rev | 62 | 150 | 15 |
| 4 | Plasmid | vector pSB1C3/rfp | Vector/RFP_for | P2-V1 | 64 | 2072 | 60 |
Table 1: The table shows the four different Phusion PCR reactions carried out for the characterisation of the hyperspankoid promoter.
Results, Discussion and Conclusion
Please refer to: 8.09.2010 for result, discussion and conclusion.
 
|
 "
"