Team:UNAM-Genomics Mexico/Modules/In vivo
From 2010.igem.org
Kurupaclau (Talk | contribs) |
Kurupaclau (Talk | contribs) |
||
| Line 27: | Line 27: | ||
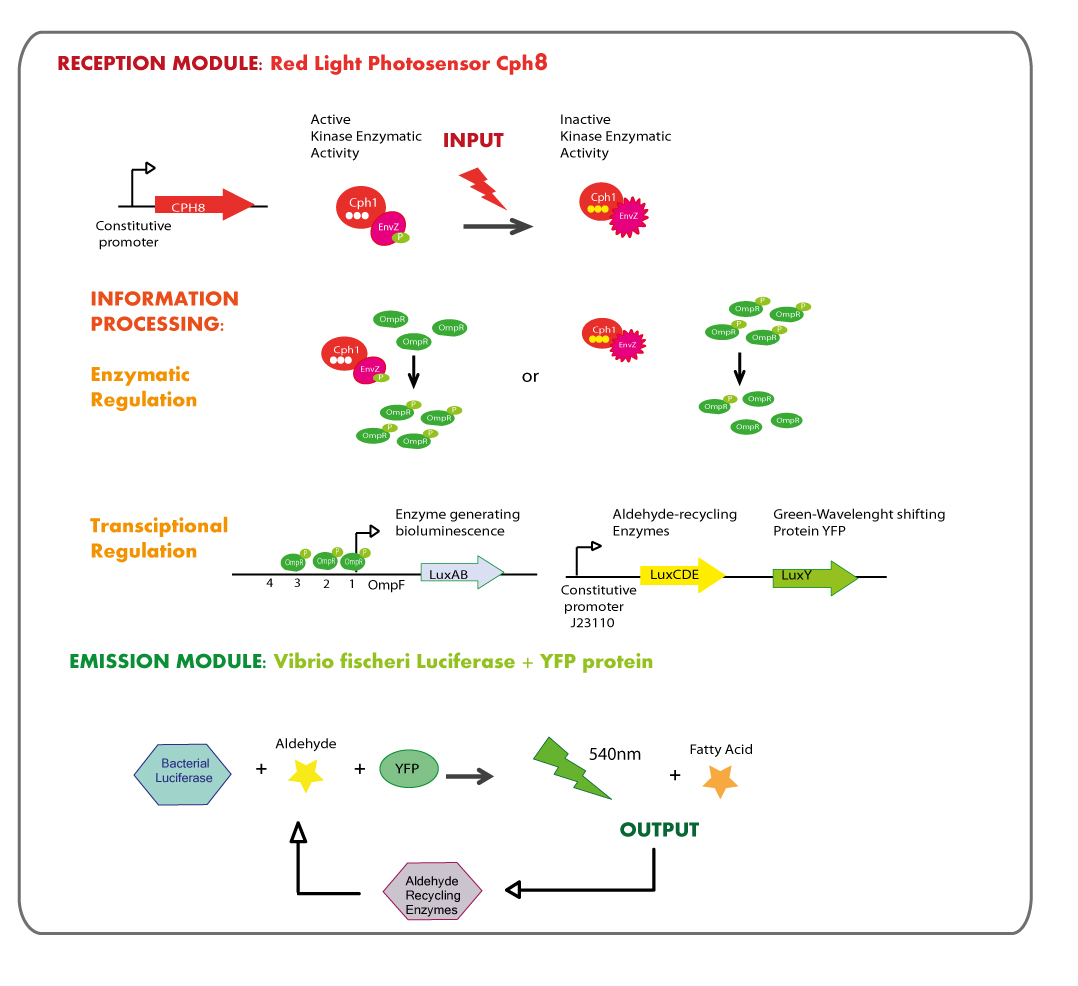
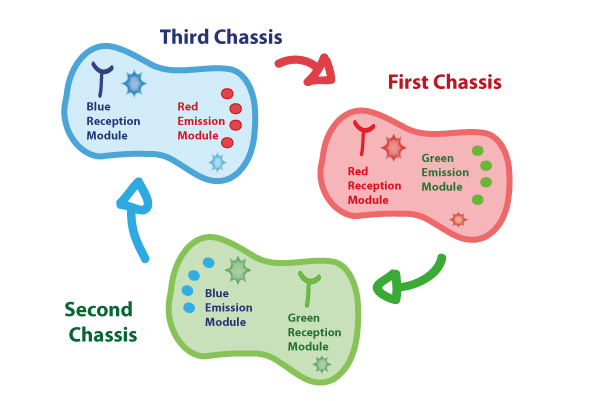
In order to enable the light based communication between bacteria, we have designed a tertiary cycle of different bacteria chassis, assembling each module of reception and emission to construct a light-based feedback loop of red-green-blue light, which will make the proof of concept of communication over distance and proper signal decoding. | In order to enable the light based communication between bacteria, we have designed a tertiary cycle of different bacteria chassis, assembling each module of reception and emission to construct a light-based feedback loop of red-green-blue light, which will make the proof of concept of communication over distance and proper signal decoding. | ||
| - | + | ==First Chassis== | |
[[Image:InRED-outGreen.jpg|thumb|center|680px| '''First Chassis''': Red Light reception module coupled to Green Light emitter module.]] | [[Image:InRED-outGreen.jpg|thumb|center|680px| '''First Chassis''': Red Light reception module coupled to Green Light emitter module.]] | ||
| - | + | ==Second Chassis== | |
[[Image:InGreen-outBlue.jpg|thumb|center|680px| '''Second Chassis:''' Green Light reception module coupled to Blue Light emitter module.]] | [[Image:InGreen-outBlue.jpg|thumb|center|680px| '''Second Chassis:''' Green Light reception module coupled to Blue Light emitter module.]] | ||
| - | + | ==Third Chassis== | |
[[Image:InBlue-outRED.jpg|thumb|center|680px| '''Third Chassis:''' Blue Light reception module coupled to Red Light emitter module.]] | [[Image:InBlue-outRED.jpg|thumb|center|680px| '''Third Chassis:''' Blue Light reception module coupled to Red Light emitter module.]] | ||
| Line 45: | Line 45: | ||
| - | == OBJECTIVES == | + | =====OBJECTIVES===== |
| Line 57: | Line 57: | ||
| - | == LovTAP design == | + | =====LovTAP design===== |
We decided to synthesize the LovTAP photosensor that in comparison with the Part:BBa_K191003 that is already at the registry, has the following differences: | We decided to synthesize the LovTAP photosensor that in comparison with the Part:BBa_K191003 that is already at the registry, has the following differences: | ||
| - | 1. The 2 PstI restriction sites were removed from the coding region of LovTAP. | + | |
| - | 2. We included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne [2]. With this mutation LovTAP should stay in the active form for longer, under light induction. For more details visit the LovTAP entry at the registry page. | + | 1. The 2 PstI restriction sites were removed from the coding region of LovTAP. |
| + | |||
| + | 2. We included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne [2]. With this mutation LovTAP should stay in the active form for longer, under light induction. | ||
| + | |||
| + | For more details visit the LovTAP entry at the registry page. | ||
3. The part does not include a promoter. | 3. The part does not include a promoter. | ||
| Line 71: | Line 75: | ||
| - | == E.coli Strain Mutant == | + | =====E.coli Strain Mutant===== |
As the transcriptional response regulated by LovTAP might also be generated by the trpR repressor in E.coli in tryptophan rich conditions , we looked for an Escherichia coli strain mutant in trpR to avoid the cross-talk of the endogenous function of this gene with our system. | As the transcriptional response regulated by LovTAP might also be generated by the trpR repressor in E.coli in tryptophan rich conditions , we looked for an Escherichia coli strain mutant in trpR to avoid the cross-talk of the endogenous function of this gene with our system. | ||
| Line 94: | Line 98: | ||
| - | == LovTAP expression Levels == | + | =====LovTAP expression Levels===== |
We got in touch with Dr. Devin Strickland the designer of LovTAP, in order to get some advices. Analyzing some experimental results reported in his dissertation and in the published paper : Light-activated DNA binding in a designed allosteric protein, it seems that at high expression levels of LovTAP, the behavior in the dark versus the light state is the same. So that, the regulation by light becomes not functional, thus we planned to test LovTAP under three different weak constitutive promoters (J23117,J23114 and J23105), expecting that it works well. | We got in touch with Dr. Devin Strickland the designer of LovTAP, in order to get some advices. Analyzing some experimental results reported in his dissertation and in the published paper : Light-activated DNA binding in a designed allosteric protein, it seems that at high expression levels of LovTAP, the behavior in the dark versus the light state is the same. So that, the regulation by light becomes not functional, thus we planned to test LovTAP under three different weak constitutive promoters (J23117,J23114 and J23105), expecting that it works well. | ||
| - | == trpL promoter == | + | =====trpL promoter===== |
The LovTAP system is composed of two modules, the light-sensitive input module is the LOV2 domain of Avena sativa phototropin 1 (AsLOV2). LOV domains absorb light through a flavin cofactor, photochemically form a covalent bond between the chromophore and a cysteine residue in the protein. The output module is the DNA binding domain of the bacterial transcription factor trp repressor (TrpR). So that, LovTAP activated can bind its operator DNA as a homodimer thus repressing transcription. | The LovTAP system is composed of two modules, the light-sensitive input module is the LOV2 domain of Avena sativa phototropin 1 (AsLOV2). LOV domains absorb light through a flavin cofactor, photochemically form a covalent bond between the chromophore and a cysteine residue in the protein. The output module is the DNA binding domain of the bacterial transcription factor trp repressor (TrpR). So that, LovTAP activated can bind its operator DNA as a homodimer thus repressing transcription. | ||
Revision as of 00:11, 27 October 2010

Coupling together Biological Chassis
In order to enable the light based communication between bacteria, we have designed a tertiary cycle of different bacteria chassis, assembling each module of reception and emission to construct a light-based feedback loop of red-green-blue light, which will make the proof of concept of communication over distance and proper signal decoding.
First Chassis
Second Chassis
Third Chassis
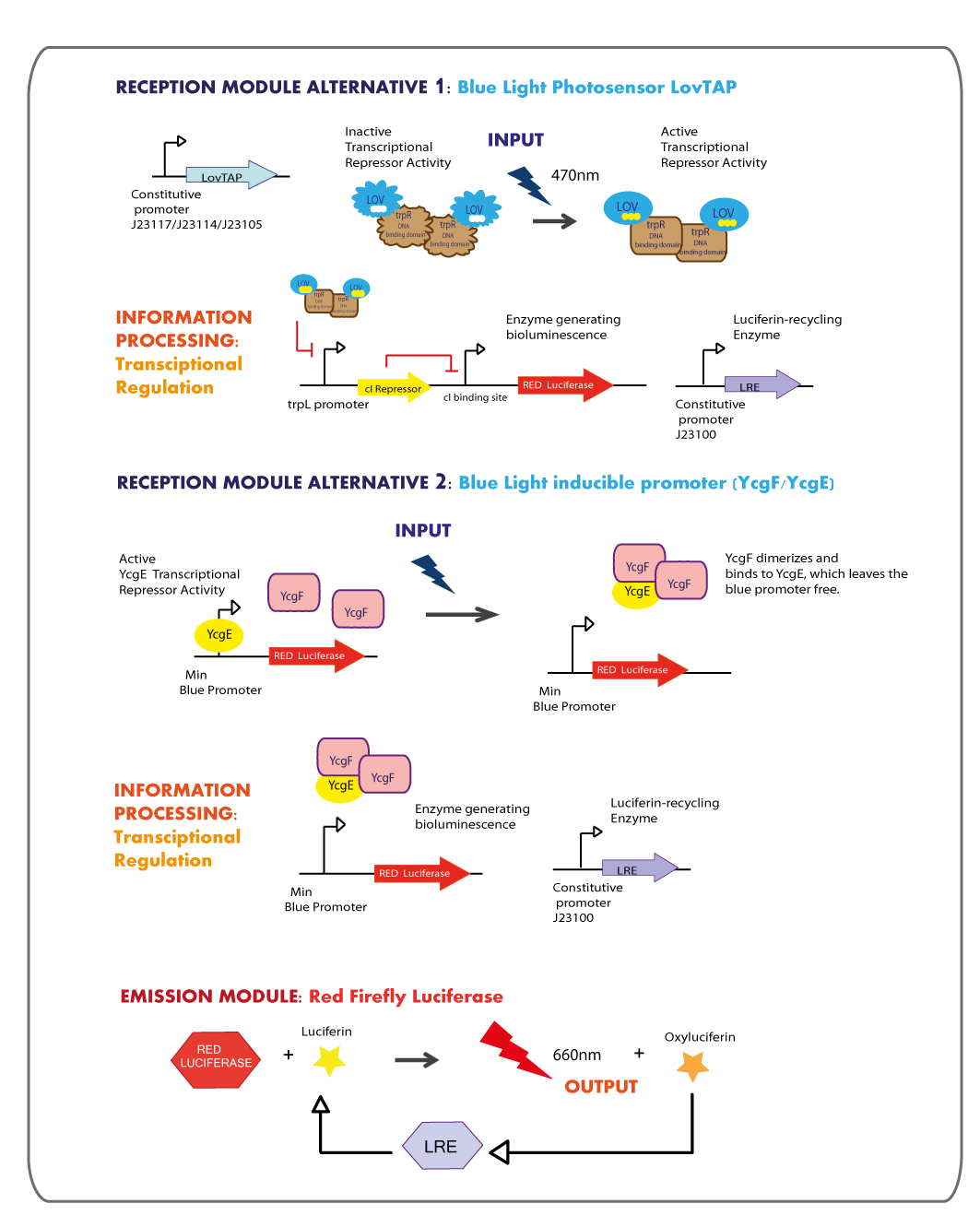
Reception module: Blue Light Photosensor LovTAP
Working in this chassis, we decided to use as blue light receptors: LovTAP photosensor and the native blue light inducible promoter from E.coli.
OBJECTIVES
- Construct an improved version of the LovTAP photosensor device under the regulation of different constitutive promoters, due to the previous version of LovTAP designed by 2009 Lausanne team didn´t have a differential behavior under the inverting regulator sensitive to LacI and CAP protein, their results showed that the expression levels of LovTAP didn’t show differences to the induction with IPTG. As well, we included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne. With this mutation LovTAP should react faster and the conformational change should be more stable (the protein stays in the active form for longer, under light induction).
- Characterize LovTAP reporter systems, both repressor and activator activity.
The structure of the device is designed as follows:
LovTAP design
We decided to synthesize the LovTAP photosensor that in comparison with the Part:BBa_K191003 that is already at the registry, has the following differences:
1. The 2 PstI restriction sites were removed from the coding region of LovTAP.
2. We included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne [2]. With this mutation LovTAP should stay in the active form for longer, under light induction.
For more details visit the LovTAP entry at the registry page.
3. The part does not include a promoter.
4. The RBS was changed from a strong (Part: BBa_B0030) to a medium strength (Part:BBa_B0032).
5. The stop codon tga was changed for two taa.
E.coli Strain Mutant
As the transcriptional response regulated by LovTAP might also be generated by the trpR repressor in E.coli in tryptophan rich conditions , we looked for an Escherichia coli strain mutant in trpR to avoid the cross-talk of the endogenous function of this gene with our system. After a careful look at the literature, we found that Dr. Charles Yanofsky had the mutant, so we sent him an e-mail, asking him to provide us the mutant.
The features of the mutant are:
1.Identification number: CY15001 2.Sex(Hfr,F+,F-,or F'): F-
5 Mutations:
•lamda- (lambda lysogen deletion)
•IN(rrnD-rrnE)1 (Inverts the region between rrnD and rrnE)
•rph-1 (RNase PH )
•tnaA5 (tryptophanase)
•trpR55 (repressor trpR)
LovTAP expression Levels
We got in touch with Dr. Devin Strickland the designer of LovTAP, in order to get some advices. Analyzing some experimental results reported in his dissertation and in the published paper : Light-activated DNA binding in a designed allosteric protein, it seems that at high expression levels of LovTAP, the behavior in the dark versus the light state is the same. So that, the regulation by light becomes not functional, thus we planned to test LovTAP under three different weak constitutive promoters (J23117,J23114 and J23105), expecting that it works well.
trpL promoter
The LovTAP system is composed of two modules, the light-sensitive input module is the LOV2 domain of Avena sativa phototropin 1 (AsLOV2). LOV domains absorb light through a flavin cofactor, photochemically form a covalent bond between the chromophore and a cysteine residue in the protein. The output module is the DNA binding domain of the bacterial transcription factor trp repressor (TrpR). So that, LovTAP activated can bind its operator DNA as a homodimer thus repressing transcription.
The DNA operator region where trp repressor binds was annotated wrongly in the 2009 EPF-Lausanne’s wiki as trpO promoter, but when searching for it in RegulonDB a very well annotated Escherichia coli transcriptional regulation data base, we found that the actually region where trp repressor binds is trpL promoter not trpO.
Knowing that we synthesized only trpLp’s functional elements as an oligonucleotide to introduce by PCR into a selected plasmid as follows:
Preffix +Promoter(functional elements only)+EcoRI
gaattcgcggccgcttctagag gctgttgacaattaatcatcgaactagttaactagtacgcaag cggaattccg
The primer is the reverse complementary of that sequence so we can use it as a reverse primer, which we used along with a suffix forward to introduce the trpL promoter into pSB3K3 plasmid by PCR.
Tertiary cycle
With the previous chassis assembled, the interchange and processing of information go through an activation signaling cascade, that is illustrated in the next image:
iGEM
iGEM is the International Genetically Engineered Machines Competition, held each year at MIT and organized with support of the Parts Registry. See more here.Synthetic Biology
This is defined as attempting to manipulate living objects as if they were man-made machines, specifically in terms of genetic engineering. See more here.Genomics
We are students on the Genomic Sciences program at the Center for Genomic Sciences of the National Autonomous University of Mexico, campus Morelos. See more here.This site is best viewed with a Webkit based Browser (eg: Google's Chrome, Apple's Safari),
Trident based (Microsoft's Internet Explorer) or Presto based (Opera) are not currently supported. Sorry.
 "
"