Team:Imperial College London/Lab Diaries/Vectors team
From 2010.igem.org
(→Week 13) |
|||
| Line 19: | Line 19: | ||
|} | |} | ||
| - | === | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" |
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 6 | ||
| + | |- | ||
| + | | | ||
| Line 97: | Line 100: | ||
</html> | </html> | ||
| - | + | '''Thursday, August 12''' | |
* Annealing the forward and reverse strands of the '''dif XP''' oligo | * Annealing the forward and reverse strands of the '''dif XP''' oligo | ||
The forward and reverse strands of the 5' dif site with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by first heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight. | The forward and reverse strands of the 5' dif site with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by first heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight. | ||
| - | + | '''Friday, August 13''' | |
* Restriction digest of '''dif XP''' | * Restriction digest of '''dif XP''' | ||
| Line 109: | Line 112: | ||
The cut oligo was PCR purified in order to get rid of any contaminants. PCR purification gets rid of short pieces of DNA which are less than about 40 base pairs. | The cut oligo was PCR purified in order to get rid of any contaminants. PCR purification gets rid of short pieces of DNA which are less than about 40 base pairs. | ||
| - | * Gel Analysis of | + | * Gel Analysis of 'dif XP' |
| + | |} | ||
| - | === | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" |
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 7 | ||
| + | |- | ||
| + | | | ||
<html> | <html> | ||
| Line 216: | Line 223: | ||
| - | + | '''Monday, August 16''' | |
*Restriction digest of '''5' ins [K143008]''' | *Restriction digest of '''5' ins [K143008]''' | ||
| Line 227: | Line 234: | ||
The PCR amplified vector was purified in order to get rid of any contaminants. For example, short pieces of DNA like the primers. | The PCR amplified vector was purified in order to get rid of any contaminants. For example, short pieces of DNA like the primers. | ||
| - | + | '''Tuesday, August 17''' | |
*Gel extraction and purification of '''5' ins ES''' | *Gel extraction and purification of '''5' ins ES''' | ||
| Line 250: | Line 257: | ||
Since these are both inserts they were gel extracted and purified. PCR purification is not carried out for inserts since they are small and would therefore be lost during the process. | Since these are both inserts they were gel extracted and purified. PCR purification is not carried out for inserts since they are small and would therefore be lost during the process. | ||
| - | + | '''Wednesday, August 18''' | |
* Replica plating and colony PCR of 5' ins and dif in pSB1C3 (bench ligation) | * Replica plating and colony PCR of 5' ins and dif in pSB1C3 (bench ligation) | ||
| Line 256: | Line 263: | ||
pveg and SpecR-T (the front inserts) were ligated overnight with pSB1C3 (the vector). | pveg and SpecR-T (the front inserts) were ligated overnight with pSB1C3 (the vector). | ||
| - | + | '''Thursday, August 19''' | |
*Transformation of E.Coli with the overnights ligates | *Transformation of E.Coli with the overnights ligates | ||
| Line 262: | Line 269: | ||
#'''pveg and SpecR-T in pSB1C3''' | #'''pveg and SpecR-T in pSB1C3''' | ||
| - | + | '''Friday, August 20''' | |
*Replica plating and colony PCR of both transformations from yesterday. | *Replica plating and colony PCR of both transformations from yesterday. | ||
| Line 268: | Line 275: | ||
*Annealing the forward and reverse strands of the dif P ES oligo | *Annealing the forward and reverse strands of the dif P ES oligo | ||
The forward and reverse strands of the 3' dif Pme1 sites with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight. | The forward and reverse strands of the 3' dif Pme1 sites with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight. | ||
| + | |} | ||
| - | === | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" |
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 8 | ||
| + | |- | ||
| + | | | ||
<html> | <html> | ||
<table width="850px" border="0"> | <table width="850px" border="0"> | ||
| Line 388: | Line 399: | ||
</html> | </html> | ||
| - | === | + | |} |
| + | |||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 9 | ||
| + | |- | ||
| + | | | ||
<html> | <html> | ||
<table width="850px" border="0"> | <table width="850px" border="0"> | ||
| Line 484: | Line 500: | ||
</table> | </table> | ||
</html> | </html> | ||
| + | |} | ||
| - | === | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" |
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 10 | ||
| + | |- | ||
| + | | | ||
<html> | <html> | ||
| Line 597: | Line 617: | ||
| - | === | + | |} |
| + | |||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 11 | ||
| + | |- | ||
| + | | | ||
<html> | <html> | ||
| Line 700: | Line 725: | ||
</table> | </table> | ||
</html> | </html> | ||
| + | |} | ||
| - | === | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" |
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 12 | ||
| + | |- | ||
| + | | | ||
<html> | <html> | ||
<table width="850px" border="0"> | <table width="850px" border="0"> | ||
| Line 814: | Line 843: | ||
</html> | </html> | ||
| - | === | + | |} |
| + | |||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Week 13 | ||
| + | |- | ||
| + | | | ||
<html> | <html> | ||
<table width="850px" border="0"> | <table width="850px" border="0"> | ||
| Line 926: | Line 960: | ||
</table> | </table> | ||
</html> | </html> | ||
| - | + | |} | |
{| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
|style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|AmyE Vector | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|AmyE Vector | ||
Revision as of 02:15, 25 October 2010
| Lab Diaries | Overview | Surface Protein Team | XylE Team | Vectors Team | Modelling Team |
| Here are the technical diaries for our project. We've split them up into three lab teams and the modelling team. We think it's really important that absolutely anyone can find out what we've been doing. For a really detailed look at what we did, and when, you've come to the right place! | |
| Objectives |
|
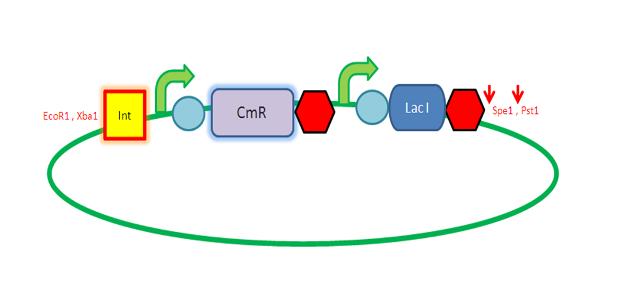
We are assembling the AmyE and PyrD vectors in order to transform B. Subtilis with our parts. Once completed, these vectors will be reusable and can then be used to introduce any relevant piece of DNA directly into B.subtilis genome. The vectors will be used both for the final assembly and for testing constructs. |
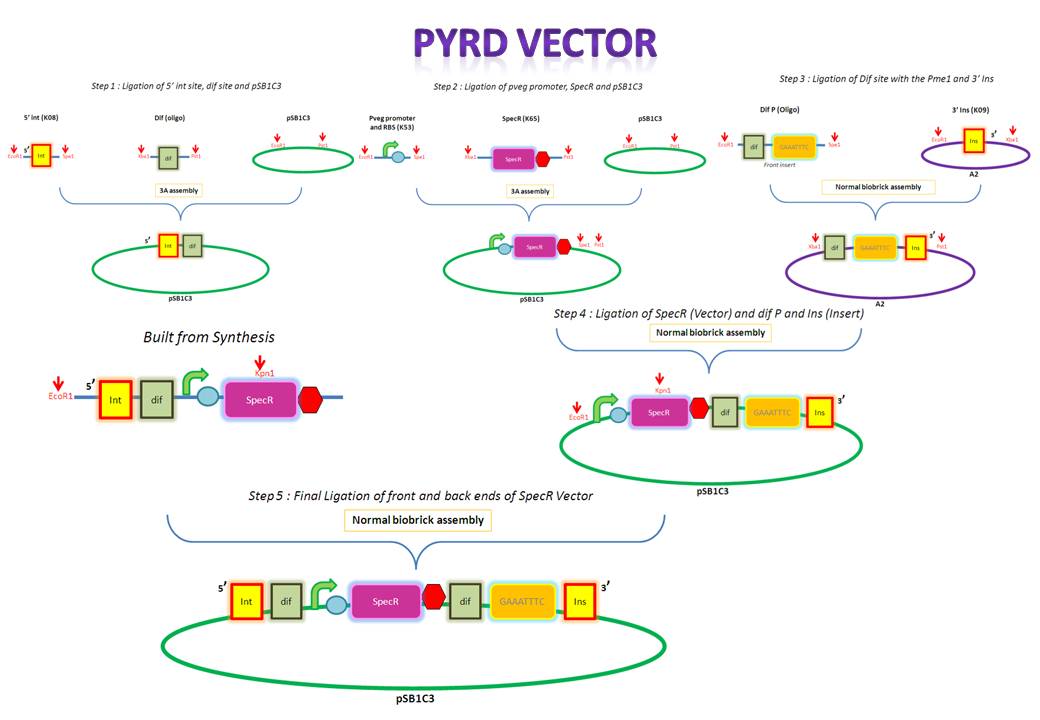
| PyrD Vector |
| Week 6 | |||||||||||||||||||||
|
Thursday, August 12
The forward and reverse strands of the 5' dif site with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by first heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight. Friday, August 13
After annealing the two strands, the oligo was cut with XbaI and PstI to obtain overhangs that would later ligate with the compatible overhangs of a cut vector.
The cut oligo was PCR purified in order to get rid of any contaminants. PCR purification gets rid of short pieces of DNA which are less than about 40 base pairs.
|
| Week 7 | ||||||||||||||||||
|
K143008 is the 5' integration site for the PyrD vector. This will be used as a front insert together with the dif XP for the pSB1C3 vector.
The pSB1C3 vector backbone from the registry was amplified with the use of SB3 and SB2a primers. Submission of parts to the registry requires them to be in a pSB1C3 vector therefore any parts to be submitted will be inserted into this vector.
The PCR amplified vector was purified in order to get rid of any contaminants. For example, short pieces of DNA like the primers. Tuesday, August 17
The digested 5' ins was first analyzed on the gel to verify it's size and then extracted for purification. The 5' ins was gel purified in order to extract only the relevant piece of DNA.
The pSB1C3 vector was digested so that it would contain compatible overhangs for ligation with inserts.
The digested pSB1C3 was re-purified in order to get rid of any contaminant DNA that arose during the digestion.
pveg (promoter and RBS) and Spec-T (Spectinomycin with a terminator) were digested in preparation for 3A assembly.
The digested 5' ins and dif (the front inserts) were ligated overnight with pSB1C3 (the vector). A bench ligation and an overnight ligation were set up.
E.Coli was transformed via the chemical method using the bench ligate.
Since these are both inserts they were gel extracted and purified. PCR purification is not carried out for inserts since they are small and would therefore be lost during the process. Wednesday, August 18
pveg and SpecR-T (the front inserts) were ligated overnight with pSB1C3 (the vector). Thursday, August 19
Friday, August 20
The forward and reverse strands of the 3' dif Pme1 sites with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight. |
| Week 8 | |||||||||||||||||||||
|
|
| Week 9 | ||||||||||||||||||
|
|
| Week 10 | ||||||||||||||||||
|
|
| Week 11 | ||||||||||||||||||
|
|
| Week 12 | |||||||||||||||||||||
|
|
| Week 13 | |||||||||||||||||||||
|
|
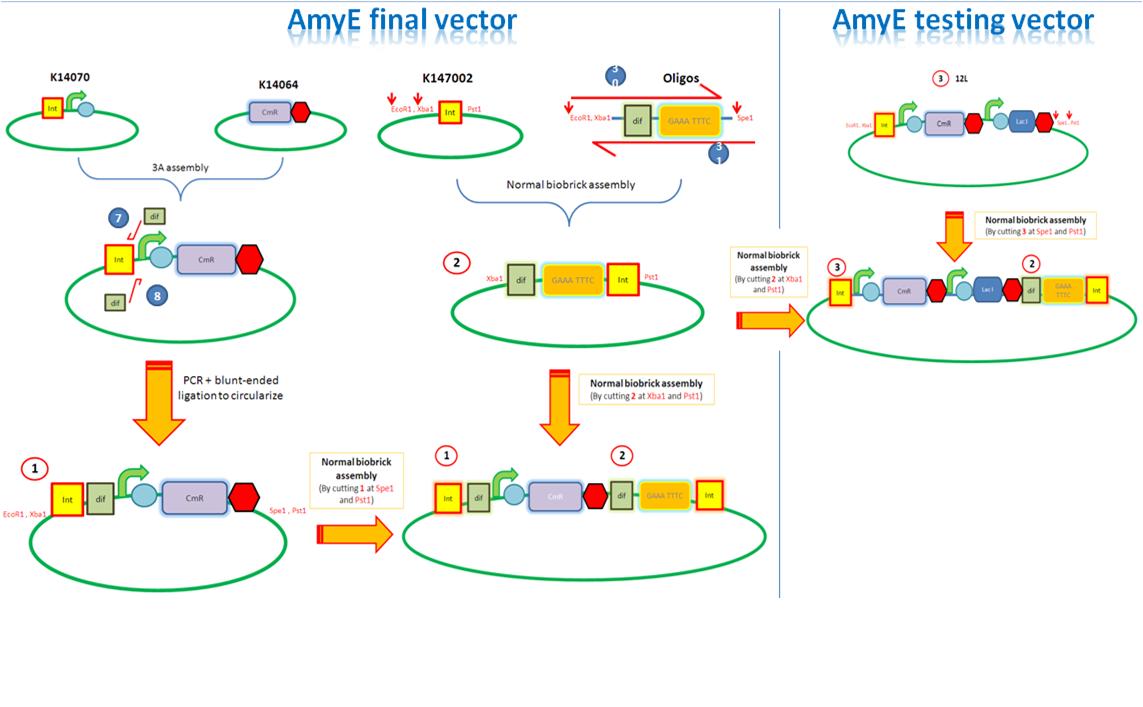
| AmyE Vector |
|
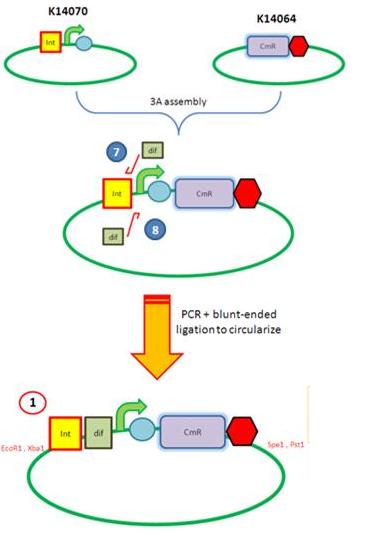
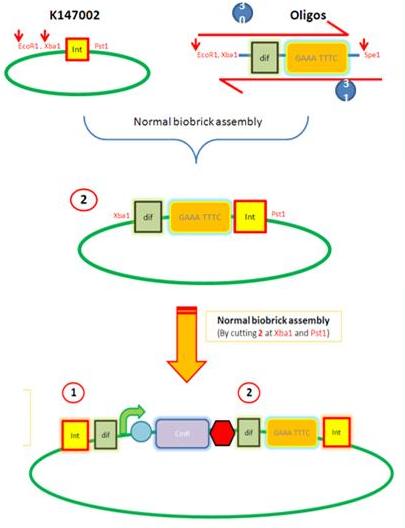
Starting from the top, we are assembling the first two fragments (K14070 and K14064) and K147002 with our oligos to add in a Dif site. Two dif sites on either side of resistance cassettes can be used to later excise antibiotic resistance from our final constructs.
Next Steps:
K14002 and oligos
Next Steps:
|
| Schedule & Lab Notes |
| Week 7 | ||||||||||||||||||
|
|
| Week 8 | |||||||||||||||||||||
|
|
| Week 9 | ||||||||||||||||||
|
|
| Week 10 |
|
|
| Week 11 |
|
|
| Week 12 |
|
|
| Week 13 | ||||||||||||||||||
|
|
 "
"