Team:Lethbridge/Results
From 2010.igem.org
Liszabruder (Talk | contribs) |
Liszabruder (Talk | contribs) |
||
| Line 155: | Line 155: | ||
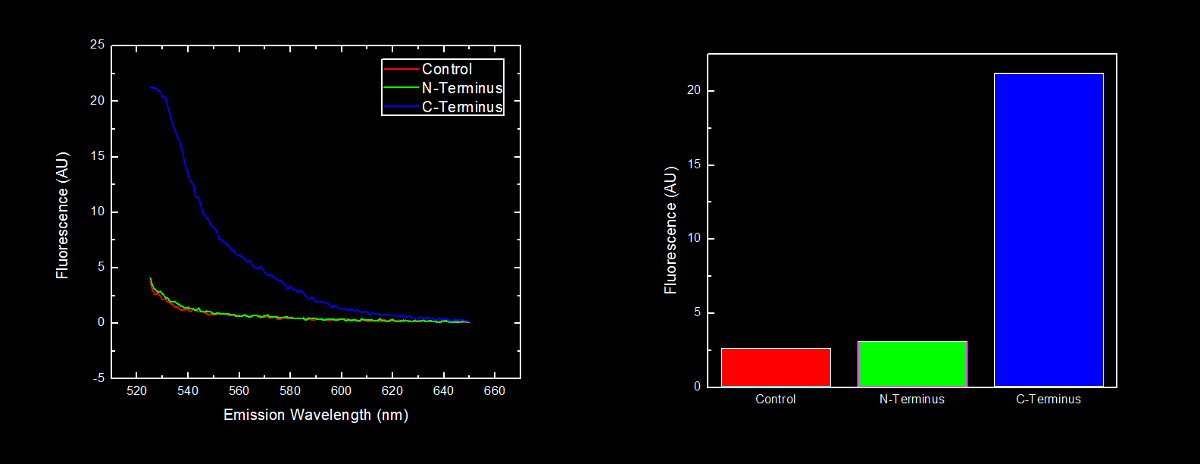
In order to further characterize the C-terminal and N-terminal oligoarginine tag (BioBricks <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K249005" target="new"><font color="green">BBa_K249005</font></a></html> and <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K249004" target="new"><font color="green">BBa_K249004</font></a></html> respectively) and investigate the effect their placement on protein stability, yellow fluorescent proteins (YFP) with the oligoarginine fused to either the C-terminus (<html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331023" target="new"><font color="green">BBa_K331023</font></a></html>) or N-terminus (<html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331022" target="new"><font color="green">BBa_K331022</font></a></html>) (and preceded by a ribosomal binding site – <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0034" target="new"><font color="green">B0034</font></a></html>) were synthesized. We used our <html><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Protocols#Assembly_of_BioBricks_using_the_Red.2FWhite_3-Antibiotic_Assembly_Method"><font color="green"> Red/White 3-Antibiotic assembly method</font></a></html> to add a tetracycline repressible promoter (<html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_R0010" target="new"><font color="green">BBa_R0010</font></a></html>) for constitutive expression of the fusion protein. This addition generated BioBricks <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331031" target="new"><font color="green">BBa_K331031</font></a></html> and <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331030" target="new"><font color="green">BBa_K331030</font></a></html> for the C-terminal tagged and N-terminal tagged YFP respectively. | In order to further characterize the C-terminal and N-terminal oligoarginine tag (BioBricks <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K249005" target="new"><font color="green">BBa_K249005</font></a></html> and <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K249004" target="new"><font color="green">BBa_K249004</font></a></html> respectively) and investigate the effect their placement on protein stability, yellow fluorescent proteins (YFP) with the oligoarginine fused to either the C-terminus (<html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331023" target="new"><font color="green">BBa_K331023</font></a></html>) or N-terminus (<html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331022" target="new"><font color="green">BBa_K331022</font></a></html>) (and preceded by a ribosomal binding site – <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_B0034" target="new"><font color="green">B0034</font></a></html>) were synthesized. We used our <html><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Protocols#Assembly_of_BioBricks_using_the_Red.2FWhite_3-Antibiotic_Assembly_Method"><font color="green"> Red/White 3-Antibiotic assembly method</font></a></html> to add a tetracycline repressible promoter (<html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_R0010" target="new"><font color="green">BBa_R0010</font></a></html>) for constitutive expression of the fusion protein. This addition generated BioBricks <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331031" target="new"><font color="green">BBa_K331031</font></a></html> and <html><a href="http://partsregistry.org/wiki/index.php/Part:BBa_K331030" target="new"><font color="green">BBa_K331030</font></a></html> for the C-terminal tagged and N-terminal tagged YFP respectively. | ||
<br><br> | <br><br> | ||
| - | The BioBrick containing plasmid was transformed into <i>Escherichia coli</i> DH5α cells. These cells were grown to an | + | The BioBrick containing plasmid was transformed into <i>Escherichia coli</i> DH5α cells. These cells were grown to an OD<sub>600</sub> of approximately 0.7, and diluted 1:10 with MilliQ H2O immediately prior to analysis by fluorescent spectroscopy. |
<br><br> | <br><br> | ||
| - | This dilution of cells was excited at | + | This dilution of cells was excited at 517 nm, and the emission spectra was read from 522 nm to 650 nm. Fluorescence at 524 nm (emission maxima of YFP) of control cells (<i>Escherichia coli</i> DH5α), N-terminal tagged, and C-terminal tagged YFP were compared. |
==<font color="white">Results</font>== | ==<font color="white">Results</font>== | ||
 "
"