Team:Imperial College London/Modelling/Output/Detailed Description
From 2010.igem.org
(Equations pasted) |
|||
| Line 304: | Line 304: | ||
*This model does not include any specific terms for time delays | *This model does not include any specific terms for time delays | ||
| - | + | <html> | |
| - | <CENTER><img src="https://static.igem.org/mediawiki/2010/e/e1/Enz_react5.png | + | <CENTER><img src="https://static.igem.org/mediawiki/2010/e/e1/Enz_react5.png"/></CENTER> |
</html> | </html> | ||
Revision as of 23:04, 24 October 2010
| Modelling | Overview | Detection Model | Signaling Model | Fast Response Model | Interactions |
| A major part of the project consisted of modelling each module. This enabled us to decide which ideas we should implement. Look at the Fast Response page for a great example of how modelling has made a major impact on our design! | |
| Objectives | Description | Results | Constants | MATLAB Code |
| Detailed Description | ||||||||||||||||||||||||||||||
|
The following page presents the details of the models that have been developed. Firstly, assumptions that have been exploited are explained. Then every model is presented separately as each of them has slightly different elements of the system and the interactions between them. However, there are only 3 fundamental biochemical processes that will be analysed:
1. Law of Mass ActionDuring a meeting with our advisors, it was noted that our initial models (in which it was assumed that our system obeyed Michaelis-Menten kinetics) were wrong as the assumptions made by Michaelis-Menten approximation were not obeyed by the system. A few of Michaelis-Menten assumptions were not met by our system:
Since we are continuously producing enzyme, Vmax will change. Therefore the conservation E0 = E + ES does not hold for our system.
We are producing both substrate and enzyme, so we have approximately the same amount of substrate and enzyme.
Abandoned Initial Attempts
Elements of the system
Simple models
Implementation of Michaelis-Menten kinetics
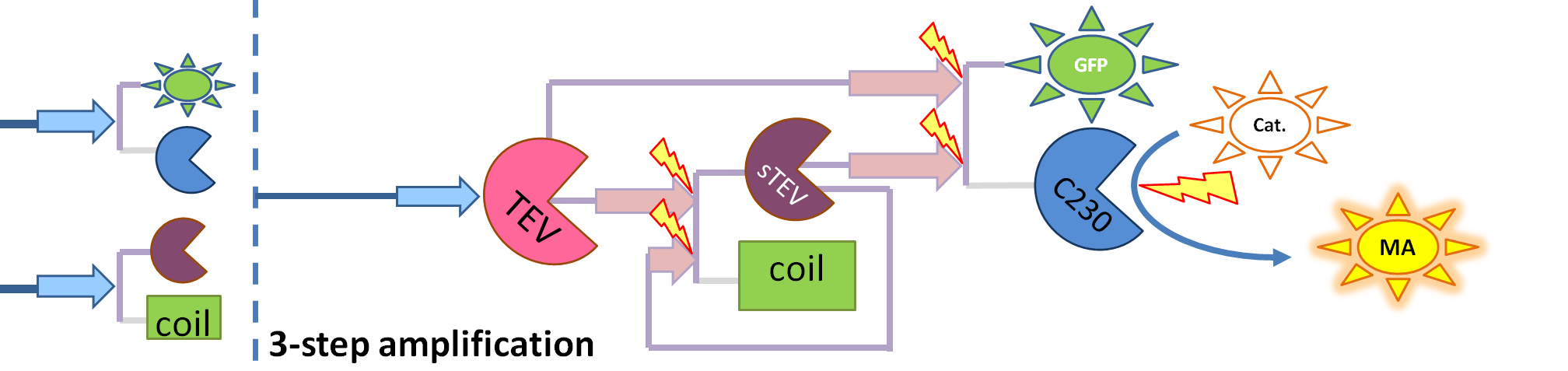
Conclusion We were not able to obtain all the necessary constants. Hence, we decided to make educated guesses about possible relative values between the constants as well as varying them and observing the change in output. As the result, we concluded that the amplification happens at each amplification level proposed. The magnitude of amplification varies depending on the constants. There is not much difference between using TEV or HIV1. Change of output During our literature research, we came across a better output, so we abandoned the idea of using GFP as an output. Instead, we are using catechol. An enzyme, dioxygenase, will be acting on the catechol, which will then result in a coloured output. Catechol will be added to the bacteria manually (i.e. the bacteria will not produce catechol). Hence, in our models dioxygenase will be treated as an output as this enzyme is the only activator of catechol in our system. This means that the change of catechol into its colourful form is dependent on the dioxygenase concentration. References
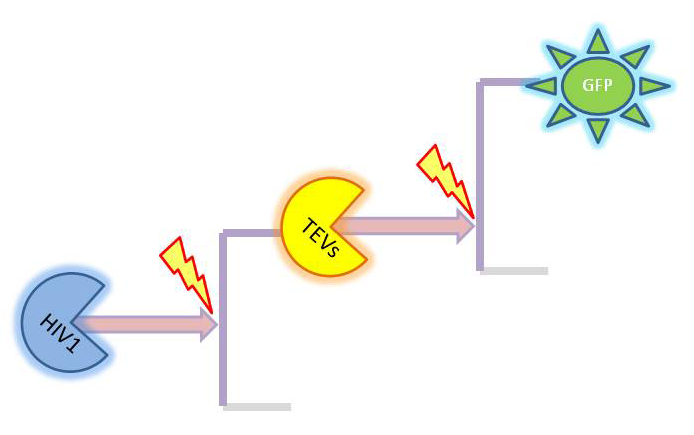
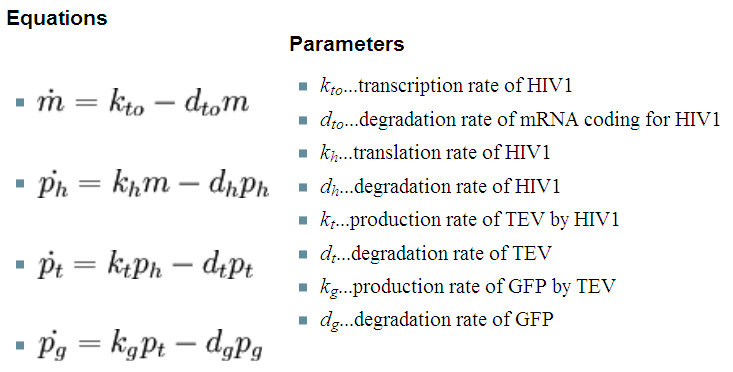
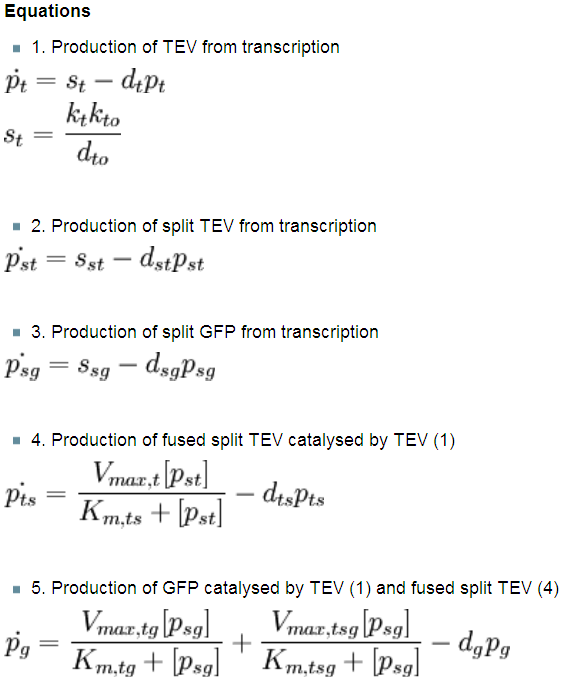
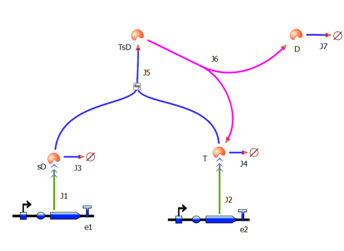
As we could not use the Michaelis-Menten simplification to model enzymatic reactions in our system, we will had to solve the problem from first principle. It meant referring to more general set of assumptions called Law of Mass Action. This allowed us to model our enzymatic reactions without making assumptions about amounts of particular species as long as the amounts are bigger than single molecule level. This resulted in bigger number of partial differential equations as there was one per each species instead of 1 per reaction. 2. Model preA: Simple production of dioxygenaseThis model was developed to illustrate 1-step amplification output. Elements of the system
Interactions between elements Initially, dioxygenase has to be transcribed and translated. This has been described using 1 ODE equation:
 The enzymatic interactions can be described in the following way:
 It is important to mention dioxygenase molecules tretramerize before becoming active enzymes. It has been very simplified in the model. Tetramerization is accounted for by simply dividing dioxygenase concentration by 4 before it acts on catechol. Differential equations The above reaction can be written in terms of differential equations:
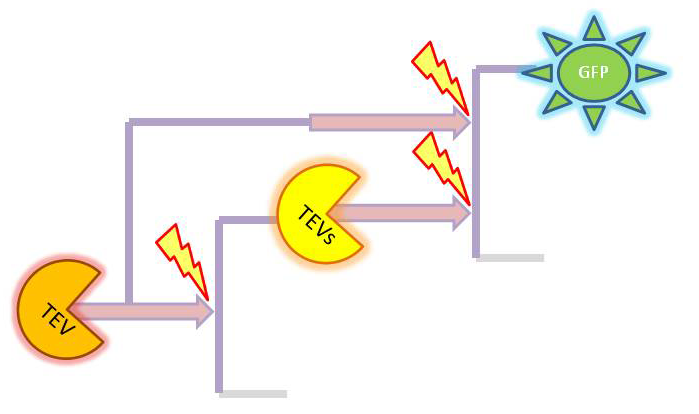
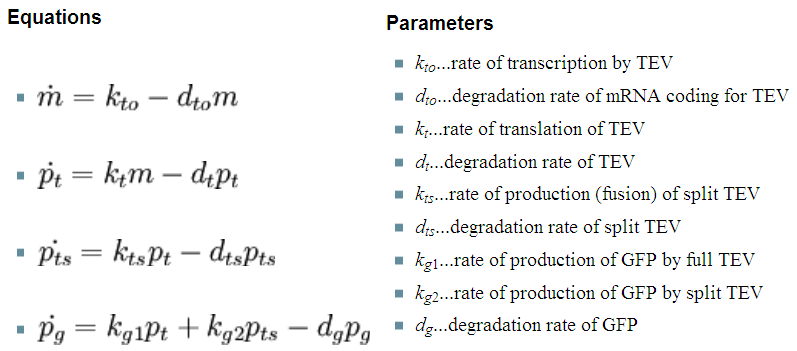
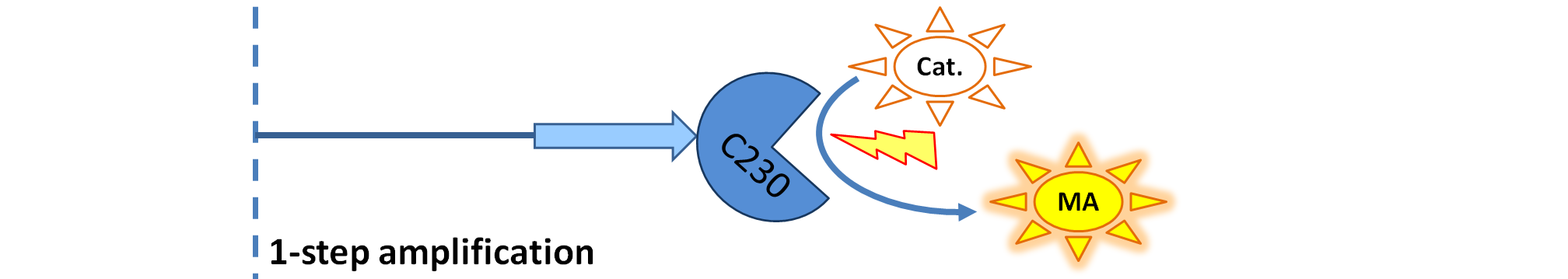
 Implementation in Matlab These equations were implemented in Matlab, using a built-in function (ode15s) which solves ordinary differential equations. For the code please refer to the Download MatLab Files section. 3. Model A: Activation of Dioxygenase by TEV enzymeThis model was developed to illustrate 1-step amplification output. Elements of the system
Interactions between elements This model includes 2 enzymatic reactions:
 Differential equations This is a simple enzymatic reaction, where TEV is the enzyme, Dioxygenase the product and split Dioxygenase the substrate. Choosing k1, k2, k3 as reaction constants, the reaction can be rewritten in these four sub-equations:
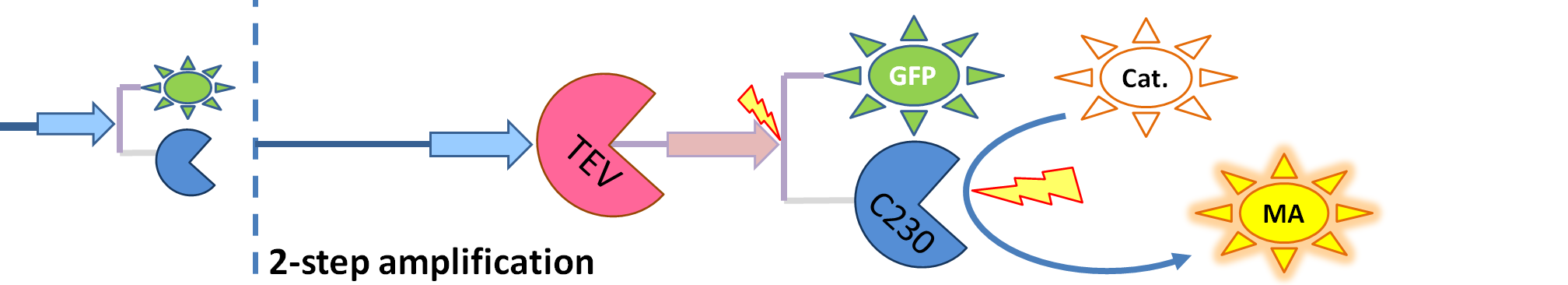
 Implementation in Matlab These equations were implemented in Matlab, using a built-in function (ode15s) which solves ordinary differential equations. For the code please refer to the Download MatLab Files section. Implementation in TinkerCell Another approach to model the amplification module would be to implement it in a program such as TinkerCell (or CellDesigner). This was used to check whether implementation in Matlab generates similar results. If happened otherwise, we would need to look for reasons for those differences in the programs. As the results, generated by Matlab were the same, only Matlab code has been developed further as it allows more flexibility, control and insight. 4. Model B: Activation of Dioxygenase by TEV or activated split TEV enzymeElements of the system
Interactions and assumptions This version includes the following features:
 Implementation in Matlab For the code please refer to the Download MatLab Files section. 5. Model C: Further improvementsThis model has not been implemented because of the conclusions that we reached from Models A and B. It would include the following features:
|
 "
"