Team:Imperial College London/Lab Diaries/Vectors team
From 2010.igem.org
| Line 16: | Line 16: | ||
__NOTOC__ | __NOTOC__ | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==Objectives== | ==Objectives== | ||
| Line 1,008: | Line 932: | ||
==AmyE Vector== | ==AmyE Vector== | ||
| - | + | [[IGEM:Imperial/2010/AmyE_Vector | Assembly of the<font color = #CA2C92> '''AmyE vector Assembly'''</font>]] | |
[[Image:IC_AmyE.JPG |750px|thumb|center|alt=A|Overveiw of AmyE assembly]] | [[Image:IC_AmyE.JPG |750px|thumb|center|alt=A|Overveiw of AmyE assembly]] | ||
| + | |||
| + | Starting from the top, we are assembling the first two fragments (K14070 and K14064) and K147002 with our oligos to add in a Dif site. Two dif sites on either side of resistance cassettes can be used to later excise antibiotic resistance from our final constructs. | ||
| + | |||
| + | [[Image:K70_n_K64.JPG |250px|thumb|right|alt=A|A : K143070 and K11064 assembly step]] | ||
| + | |||
| + | |||
| + | '''K14070 and K14064 fragments''' | ||
| + | # DNA was taken out of the reigstry | ||
| + | # Cut with restriction enzymes | ||
| + | # Run on a gel to confirm correct cutting and estimate relative ratios of DNA for ligation | ||
| + | # Ligated overnight | ||
| + | # Transformed into E.Coli to Amplify the DNA | ||
| + | # Colony PCR has been used to confirm the correct insert size. | ||
| + | |||
| + | Next Steps: | ||
| + | # Extract the DNA with a miniprep | ||
| + | # Proceed to the next step - Reverse PCR | ||
| + | |||
| + | |||
| + | [[Image:K02_and_oligos.JPG |250px|thumb|right|alt=A|B : K14002 and oligo assembly]] | ||
| + | |||
| + | |||
| + | |||
| + | '''K14002 and oligos''' | ||
| + | # Ligated two single stranded oligos together to produce Dif and insertion site | ||
| + | # Standard biobrick assembly of oligos to K14002 | ||
| + | # Ligation and transformation into E.coli competent cells (strain) | ||
| + | # Screen plated colonies for correct insertion | ||
| + | |||
| + | Next Steps: | ||
| + | # Purify the correct insert out of E.coli | ||
| + | # Next step assembly - LacI test vector and Final assembly vector | ||
| + | |||
| + | [[Image:LacI_testing_construct |250px|thumb|right|alt=A|B2 : LacI Testing Vector]] | ||
| + | |||
| + | |||
| + | '''LacI Testing vector''' | ||
| + | Currently waiting for Part B step 2 Midi-prep results to start this step. | ||
| + | |||
| + | |||
| + | ==Schedule & Lab notes== | ||
| + | ===Week 7=== | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1" | ||
| + | |||
| + | |- | ||
| + | ! Week 6 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday | ||
| + | |- | ||
| + | | MORNING | ||
| + | ||<font color = #CA2C92>'''Starting assembly of AmyE vector'''</font> | ||
| + | *<font color = #CA2C92>Restriction digestion of BB k14070 for the AmyE vector (using Eco and Spe)</font> | ||
| + | *<font color = #CA2C92>Restriction digestion of BB k14064 for the AmyE vector (using Eco and Xba)</font> | ||
| + | *<font color = #6B3FA0>Restriction digestion of 5' integration site (k08) for the PyrD vector (using Eco and Spe)</font> | ||
| + | *<font color = #6B3FA0>PCR amplification of vector backbone PSB1C3 for the PyrD vector</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis and extraction of 5' int site for PyrD vector (repetition of step due to absence of DNA during gel analysis yesterday)</font> | ||
| + | *<font color = #CA2C92>Gel purification of k70</font> | ||
| + | *<font color = #6B3FA0>Gel purification of k08</font> | ||
| + | *<font color = #CA2C92>Re-analysis of k70, k64 (for AmyE) on gel to work out ratios for ligation set up</font> | ||
| + | *<font color = #6B3FA0>Re-analysis of k08, Pme oligos and pSB1C3 (for PyrD) on gel to work out ratios for ligation set up</font> | ||
| + | *<font color = #6B3FA0>Restriction digestion of pveg promoter (k53) (using Eco and Spe) and spec cassette (k65) (using Xba and Pst) for PyrD vector</font> | ||
| + | *<font color = #6B3FA0>Restriction digestion of pSB1C3 for PyrD (using Eco and Pst)</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Check for transformed colonies (colonies that have taken up the vector with the 5'diff) and prepare for colony PCR</font> | ||
| + | *<font color = #6B3FA0>Gel purification of k53 and k65 for AmyE in preparation for ligations this afternoon</font> | ||
| + | *<font color = #6B3FA0>Gel extraction and re-analysis on gel of k53, k65 and psB1C3 for Spec casette in preparation for ligations this afternoon</font> | ||
| + | || | ||
| + | *<font color = #CA2C92>Transformation of overnight ligations of: | ||
| + | k64 and k70, k70 only, </font> | ||
| + | <font color = #6B3FA0> | ||
| + | Spec </font> | ||
| + | <font color = #6B3FA0> | ||
| + | and 5' PyrD diff</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Replica plating and colony PCR of: | ||
| + | Spec and 5' PyrD diff</font> | ||
| + | *<font color = #CA2C92>Plate wash of: | ||
| + | k64 and k70. k70 only was discarded since this was purely for a baground check</font> | ||
| + | |- | ||
| + | | AFTERNOON | ||
| + | || | ||
| + | *<font color = #6B3FA0>PCR purification of PSB1C3 vector </font> | ||
| + | *<font color = #CA2C92>PCR purification of BB k14064 digestion products</font> | ||
| + | *<font color = #CA2C92>Gel analysis of digestion products of BB k14070</font> | ||
| + | *<font color = #CA2C92>Gel extraction of digestion products of BB k14070</font> | ||
| + | || | ||
| + | *<font color = #CA2C92>Restriction digestion of k64 and subsequent PCR purification (repetition of step due to absence of DNA during gel analysis)</font> | ||
| + | *<font color = #CA2C92>Gel analysis of k70, k64 (for AmyE) to work out ligation ratios</font> | ||
| + | *<font color = #6B3FA0>Gel analysis and extraction of k53 and k65</font> | ||
| + | *<font color = #6B3FA0>Bench (1 hour) and overnight ligation of 5'diff with the pSB1C3 vector</font> | ||
| + | *<font color = #6B3FA0>Transformation of E.Coli with the bench ligated vector</font> | ||
| + | || | ||
| + | *<font color = #CA2C92>Dephosphorylation of k64 and set up of overnight ligations for k64 and k70 (vector and insert) and k64 (vector)only (for negative control; check of background)</font> | ||
| + | *<font color = #6B3FA0>Set up overnight ligations of SpecR</font> | ||
| + | || | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of colony PCR products of Spec and 5' PyrD diff</font> | ||
| + | * Annealing of diff P oligos (used for both PyrD and AmyE vectors) | ||
| + | |} | ||
| + | |||
| + | ===Week 8=== | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1" | ||
| + | |||
| + | |- | ||
| + | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday !! Saturday | ||
| + | |- | ||
| + | | MORNING | ||
| + | || | ||
| + | Set up overnight ligations for standard assembly (BBA) and 3A cloning (3A) of dif P | ||
| + | *<font color = #CA2C92>Restriction digestion of 3' integration site (K02) in the A2 vector ( Using Eco and Xba) for BBA | ||
| + | </font> | ||
| + | *<font color = #6B3FA0>Restriction digestion of 3' integration site (K09) in the AK3 vector ( Using Eco and Xba) for BBA | ||
| + | </font> | ||
| + | *<font color = #CA2C92>PCR purification of 3' integration site (K02) in the A2 vector ( Using Eco and Xba) for BBA | ||
| + | </font> | ||
| + | *<font color = #6B3FA0>PCR purification of 3' integration site (K09) in the AK3 vector ( Using Eco and Xba) for BBA | ||
| + | </font> | ||
| + | *<font color = #6B3FA0>Set up 5 ml culture of spec from colony 1 of replica plate in shaking incubator @ 37 degrees | ||
| + | </font> | ||
| + | || | ||
| + | *<font color = #CA2C92>Restriction digestion of 3' integration site (K02) in the A2 vector ( Using Xba and Pst) for 3A | ||
| + | </font> | ||
| + | *<font color = #6B3FA0>Restriction digestion of 3' integration site (K09) in the AK3 vector ( Using Xba and Pst) for 3A | ||
| + | </font> | ||
| + | || | ||
| + | * Transformations using the overnight ligations showed a lot of background. Therefore we set up ligations for K02 and K09 using 3A cloning. The results will show if this method is preferable due to less background. | ||
| + | *<font color = #CA2C92>Midiprep of CmR vector and test digest using Eco and Spe</font> | ||
| + | |||
| + | * Backbone PCR of PSB1C3 vector (1st attempt) for common use | ||
| + | |||
| + | |||
| + | || | ||
| + | *<font color = #6B3FA0>Replica plating of transformed colonies for k09 from the plate with the insert (diff P) - 45 sigle colonies were plated</font> | ||
| + | *<font color = #6B3FA0> The first 15 of the above colonies were colony PCRed using dif PES Fwd and pSB Rev</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of colony PCR from yesterday (first 15 replica plated colonies</font> | ||
| + | *<font color = #6B3FA0>Set up further colony PCR reactions for the same 15 colonies using pSB Fwd and pSB Rev primers</font> | ||
| + | * Gel analysis of pSB1C3 - after backbone PCR and after digestion (looked contaminated!) | ||
| + | * Test digests of K54 K70 Midi prep using i) EcoRI, ii) SpeI and iii) EcoRI + SpeI | ||
| + | * Screen next 20 colonies by colony PCR, use higher temperatures to avoid previous non specific annealing | ||
| + | || | ||
| + | *<font color = #6B3FA0>Miniprep of 4 overnight cultures (dif P and 3' Insert for PyrD) - colonies 4,5,7 & 9</font> | ||
| + | * Run Colony PCR results on a Gel - pick promissing candidates for mini prep. | ||
| + | |||
| + | |- | ||
| + | | AFTERNOON | ||
| + | || | ||
| + | * Gel analysis of PCR purified K02 and K09 with the insert (diff P) in between to work out ratios for the liagation | ||
| + | *<font color = #CA2C92>Dephosphorylation of digested A2 vector with 3' integration site (K02) | ||
| + | </font> | ||
| + | *<font color = #6B3FA0>Dephosphorylation of digested AK3 vector with 3' integration site (K09) | ||
| + | </font> | ||
| + | *<font color = #CA2C92>Set up overnight ligation of A2 vector with 3' integration site (K02) with diff P (insert) and A2 vector only | ||
| + | </font> | ||
| + | *<font color = #6B3FA0>Set up overnight ligation of AK3 vector with 3' integration site (K09) with diff P (insert) and AK3 vector only</font> | ||
| + | *<font color = #6B3FA0>Colony PCR and gel analysis of plated culture (from plate wash on Friday) with K64 and K70</font> | ||
| + | *<font color = #6B3FA0>Overnight 100 ml culture of spec @ 14 degrees </font> | ||
| + | || | ||
| + | * Electroporation of the 4 overnight ligations described on Thursday afternoon | ||
| + | * Gel extraction of the digestion products ( 3' integration sites - now our inserts) described this morning for 3A | ||
| + | * Gel analysis of gel extracted K02 nad K09 (inserts) with diff P (also an insert) and pSB1C3 (vector) in between | ||
| + | *<font color = #CA2C92>Set up overnight 100 ml culture of CmR vector (K64 and K70) for midiprep tomorrow</font> | ||
| + | * Set up overnight culture plates (AmpR) for the 4 electroporated cultures (colonies that survive will contain transformed cells | ||
| + | |||
| + | || | ||
| + | * PCR purification of PSB1C3 PCR amplified vector | ||
| + | * Check concentration of midi prepped CmR | ||
| + | * Run a gel to visualise the results - Gel contained pSB1C3 (PCR purified) , CmR (Midiprepped) and CmR digested (Midiprepped) | ||
| + | |||
| + | || | ||
| + | * Backbone PCR of pSB1C3 (2nd attempt), PCR purified and then digested with Eco and Pst | ||
| + | * Midipreps sent for sequencing ( Spec and CmR) | ||
| + | || | ||
| + | Backbone PCR of pSB1C3 using PFU (3rd attempt) | ||
| + | *<font color = #6B3FA0>Set up overnight 5 ml cultures for miniprepping tomorrow - 4 cultures were set up by looking at the gel this morning; 2 positive looking (4 & 7), 1 negative (5) and one containing nothing (9)</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Diagnostic digests of minipreps - Two digests : One with Spe & Pst and other with Xba & Spe</font> | ||
| + | |} | ||
| + | |||
| + | ===Week 9=== | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1" | ||
| + | |||
| + | |- | ||
| + | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday | ||
| + | |- | ||
| + | | MORNING | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best</font> | ||
| + | *Test Digest of Mini Prep K02+dif EcoRI and SpeI | ||
| + | |||
| + | || | ||
| + | *<font color = #6B3FA0>Midiprep of Colony 4 - concentration 110 ng/ul</font> | ||
| + | *Restriction digest of K02 from registrty for 3A assembly. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel purification of insert (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel analysis of vector and insert to work out ratios for ligations</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0> Transformation with overnight ligations</font> | ||
| + | *PCR amplified the PSB1C3 vector for 3A assembly | ||
| + | *Gel Purified K02 and PBB1C3 digests | ||
| + | || | ||
| + | *<font color = #6B3FA0> The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies</font> | ||
| + | *<font color = #6B3FA0> 10 individual colonies were replica plated and used for colony PCR</font> | ||
| + | *Dephosphorylate PSB1C3 using alkaline phosphotase | ||
| + | || | ||
| + | |- | ||
| + | | AFTERNOON | ||
| + | || | ||
| + | *<font color = #6B3FA0>Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow</font> | ||
| + | *Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst</font> | ||
| + | *<font color = #6B3FA0>PCR purification of vetor (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel extraction of insert after gel analysis (35 ul)</font> | ||
| + | *Midi prep of sample 8 K54 + K70 | ||
| + | || | ||
| + | *<font color = #6B3FA0>Dephosphorylation of vector</font> | ||
| + | *<font color = #6B3FA0>Set up two overnight ligations; Vector & Insert and Vector only </font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of colony PCR products</font> | ||
| + | *set up ligation reaction K02+dif using 3A method for Transformation on Monday. | ||
| + | |} | ||
| + | |||
| + | ===Week 10=== | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1" | ||
| + | |||
| + | |- | ||
| + | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday | ||
| + | |- | ||
| + | | MORNING | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best</font> | ||
| + | *Test Digest of Mini Prep K02+dif EcoRI and SpeI | ||
| + | |||
| + | || | ||
| + | *<font color = #6B3FA0>Midiprep of Colony 4 - concentration 110 ng/ul</font> | ||
| + | *Restriction digest of K02 from registrty for 3A assembly. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel purification of insert (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel analysis of vector and insert to work out ratios for ligations</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0> Transformation with overnight ligations</font> | ||
| + | *PCR amplified the PSB1C3 vector for 3A assembly | ||
| + | *Gel Purified K02 and PBB1C3 digests | ||
| + | || | ||
| + | *<font color = #6B3FA0> The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies</font> | ||
| + | *<font color = #6B3FA0> 10 individual colonies were replica plated and used for colony PCR</font> | ||
| + | *Dephosphorylate PSB1C3 using alkaline phosphotase | ||
| + | || | ||
| + | |- | ||
| + | | AFTERNOON | ||
| + | || | ||
| + | *<font color = #6B3FA0>Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow</font> | ||
| + | *Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst</font> | ||
| + | *<font color = #6B3FA0>PCR purification of vetor (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel extraction of insert after gel analysis (35 ul)</font> | ||
| + | *Midi prep of sample 8 K54 + K70 | ||
| + | || | ||
| + | *<font color = #6B3FA0>Dephosphorylation of vector</font> | ||
| + | *<font color = #6B3FA0>Set up two overnight ligations; Vector & Insert and Vector only </font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of colony PCR products</font> | ||
| + | *set up ligation reaction K02+dif using 3A method for Transformation on Monday. | ||
| + | |} | ||
| + | |||
| + | ===Week 11=== | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1" | ||
| + | |||
| + | |- | ||
| + | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday | ||
| + | |- | ||
| + | | MORNING | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best</font> | ||
| + | *Test Digest of Mini Prep K02+dif EcoRI and SpeI | ||
| + | |||
| + | || | ||
| + | *<font color = #6B3FA0>Midiprep of Colony 4 - concentration 110 ng/ul</font> | ||
| + | *Restriction digest of K02 from registrty for 3A assembly. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel purification of insert (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel analysis of vector and insert to work out ratios for ligations</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0> Transformation with overnight ligations</font> | ||
| + | *PCR amplified the PSB1C3 vector for 3A assembly | ||
| + | *Gel Purified K02 and PBB1C3 digests | ||
| + | || | ||
| + | *<font color = #6B3FA0> The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies</font> | ||
| + | *<font color = #6B3FA0> 10 individual colonies were replica plated and used for colony PCR</font> | ||
| + | *Dephosphorylate PSB1C3 using alkaline phosphotase | ||
| + | || | ||
| + | |- | ||
| + | | AFTERNOON | ||
| + | || | ||
| + | *<font color = #6B3FA0>Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow</font> | ||
| + | *Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst</font> | ||
| + | *<font color = #6B3FA0>PCR purification of vetor (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel extraction of insert after gel analysis (35 ul)</font> | ||
| + | *Midi prep of sample 8 K54 + K70 | ||
| + | || | ||
| + | *<font color = #6B3FA0>Dephosphorylation of vector</font> | ||
| + | *<font color = #6B3FA0>Set up two overnight ligations; Vector & Insert and Vector only </font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of colony PCR products</font> | ||
| + | *set up ligation reaction K02+dif using 3A method for Transformation on Monday. | ||
| + | |} | ||
| + | |||
| + | ===Week 12=== | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1" | ||
| + | |||
| + | |- | ||
| + | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday | ||
| + | |- | ||
| + | | MORNING | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best</font> | ||
| + | *Test Digest of Mini Prep K02+dif EcoRI and SpeI | ||
| + | |||
| + | || | ||
| + | *<font color = #6B3FA0>Midiprep of Colony 4 - concentration 110 ng/ul</font> | ||
| + | *Restriction digest of K02 from registrty for 3A assembly. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel purification of insert (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel analysis of vector and insert to work out ratios for ligations</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0> Transformation with overnight ligations</font> | ||
| + | *PCR amplified the PSB1C3 vector for 3A assembly | ||
| + | *Gel Purified K02 and PBB1C3 digests | ||
| + | || | ||
| + | *<font color = #6B3FA0> The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies</font> | ||
| + | *<font color = #6B3FA0> 10 individual colonies were replica plated and used for colony PCR</font> | ||
| + | *Dephosphorylate PSB1C3 using alkaline phosphotase | ||
| + | || | ||
| + | |- | ||
| + | | AFTERNOON | ||
| + | || | ||
| + | *<font color = #6B3FA0>Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow</font> | ||
| + | *Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst</font> | ||
| + | *<font color = #6B3FA0>PCR purification of vetor (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel extraction of insert after gel analysis (35 ul)</font> | ||
| + | *Midi prep of sample 8 K54 + K70 | ||
| + | || | ||
| + | *<font color = #6B3FA0>Dephosphorylation of vector</font> | ||
| + | *<font color = #6B3FA0>Set up two overnight ligations; Vector & Insert and Vector only </font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of colony PCR products</font> | ||
| + | *set up ligation reaction K02+dif using 3A method for Transformation on Monday. | ||
| + | |} | ||
| + | |||
| + | ===Week 13=== | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" border="1" | ||
| + | |||
| + | |- | ||
| + | ! Week 8 !! Monday !! Tuesday!! Wednesday !! Thursday !! Friday | ||
| + | |- | ||
| + | | MORNING | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best</font> | ||
| + | *Test Digest of Mini Prep K02+dif EcoRI and SpeI | ||
| + | |||
| + | || | ||
| + | *<font color = #6B3FA0>Midiprep of Colony 4 - concentration 110 ng/ul</font> | ||
| + | *Restriction digest of K02 from registrty for 3A assembly. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel purification of insert (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel analysis of vector and insert to work out ratios for ligations</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0> Transformation with overnight ligations</font> | ||
| + | *PCR amplified the PSB1C3 vector for 3A assembly | ||
| + | *Gel Purified K02 and PBB1C3 digests | ||
| + | || | ||
| + | *<font color = #6B3FA0> The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies</font> | ||
| + | *<font color = #6B3FA0> 10 individual colonies were replica plated and used for colony PCR</font> | ||
| + | *Dephosphorylate PSB1C3 using alkaline phosphotase | ||
| + | || | ||
| + | |- | ||
| + | | AFTERNOON | ||
| + | || | ||
| + | *<font color = #6B3FA0>Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow</font> | ||
| + | *Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector. | ||
| + | || | ||
| + | *<font color = #6B3FA0>Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst</font> | ||
| + | *<font color = #6B3FA0>PCR purification of vetor (35 ul)</font> | ||
| + | *<font color = #6B3FA0>Gel extraction of insert after gel analysis (35 ul)</font> | ||
| + | *Midi prep of sample 8 K54 + K70 | ||
| + | || | ||
| + | *<font color = #6B3FA0>Dephosphorylation of vector</font> | ||
| + | *<font color = #6B3FA0>Set up two overnight ligations; Vector & Insert and Vector only </font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Both ligations ('''Insert & Vector''' and '''Vector only''') were plated in CmR and incubated overnight</font> | ||
| + | || | ||
| + | *<font color = #6B3FA0>Gel analysis of colony PCR products</font> | ||
| + | *set up ligation reaction K02+dif using 3A method for Transformation on Monday. | ||
| + | |} | ||
Revision as of 18:48, 23 October 2010
| Lab Diaries | Overview | Surface Protein Team | XylE Team | Vectors Team | Modelling Team |
| Here are the technical diaries for our project. We've split them up into three lab teams and the modelling team. We think it's really important that absolutely anyone can find out what we've been doing. For a really detailed look at what we did, and when, you've come to the right place! | |
| Week 6 | Monday | Tuesday | Wednesday | Thursday | Friday |
| Morning |
|
||||
| Afternoon |
|
|
Thursday, August 12
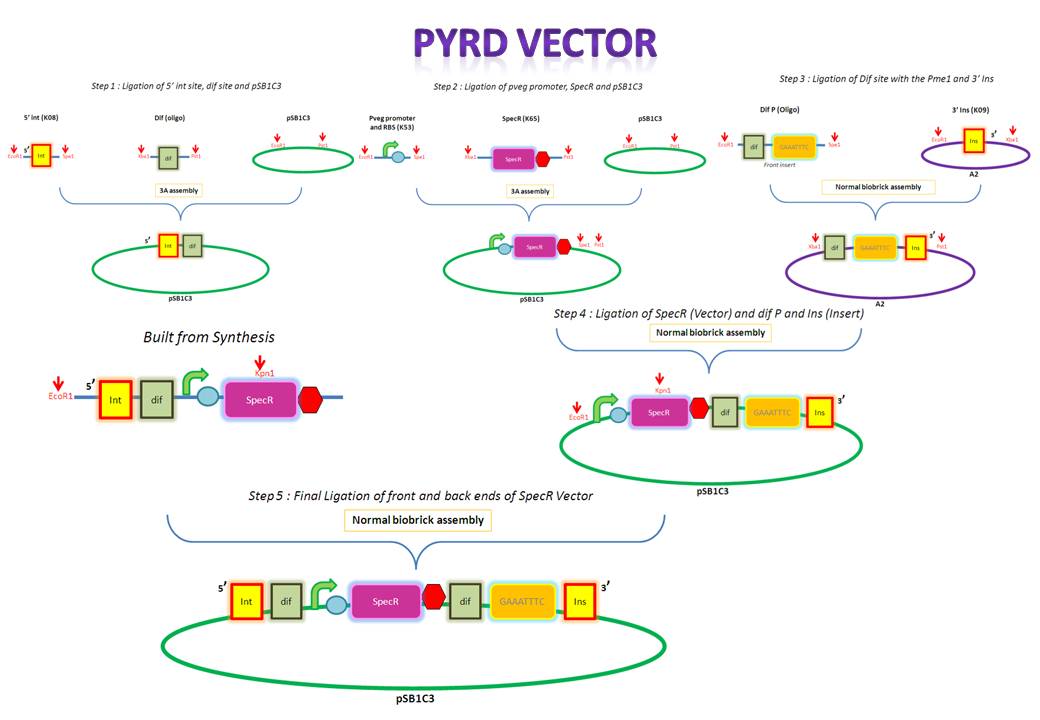
- Annealing the forward and reverse strands of the dif XP oligo
The forward and reverse strands of the 5' dif site with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by first heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight.
Friday, August 13
- Restriction digest of dif XP
After annealing the two strands, the oligo was cut with XbaI and PstI to obtain overhangs that would later ligate with the compatible overhangs of a cut vector.
- PCR purification of the cut dif XP
The cut oligo was PCR purified in order to get rid of any contaminants. PCR purification gets rid of short pieces of DNA which are less than about 40 base pairs.
- Gel Analysis of dif XP
Week 7
| Week 7 | Monday | Tuesday | Wednesday | Thursday | Friday |
| Morning |
|
|
|
|
|
| Afternoon |
|
|
|
|
Monday, August 16
- Restriction digest of 5' ins [K143008]
K143008 is the 5' integration site for the PyrD vector. This will be used as a front insert together with the dif XP for the pSB1C3 vector.
- PCR amplification of pSB1C3
The pSB1C3 vector backbone from the registry was amplified with the use of SB3 and SB2a primers. Submission of parts to the registry requires them to be in a pSB1C3 vector therefore any parts to be submitted will be inserted into this vector.
- PCR purification of pSB1C3
The PCR amplified vector was purified in order to get rid of any contaminants. For example, short pieces of DNA like the primers.
Tuesday, August 17
- Gel extraction and purification of 5' ins ES
The digested 5' ins was first analyzed on the gel to verify it's size and then extracted for purification. The 5' ins was gel purified in order to extract only the relevant piece of DNA.
- Restriction digest of pSB1C3
The pSB1C3 vector was digested so that it would contain compatible overhangs for ligation with inserts.
- PCR purification of pSB1C3 EP
The digested pSB1C3 was re-purified in order to get rid of any contaminant DNA that arose during the digestion.
- Restriction digests of pveg and Spec-T
pveg (promoter and RBS) and Spec-T (Spectinomycin with a terminator) were digested in preparation for 3A assembly.
- Ligation of 5' ins (ES) and dif (XP) with pSB1C3 (EP)
The digested 5' ins and dif (the front inserts) were ligated overnight with pSB1C3 (the vector). A bench ligation and an overnight ligation were set up.
- Transformation of E.Coli with bench ligate
E.Coli was transformed via the chemical method using the bench ligate.
- Gel extraction and purification of pveg and Spec-T
Since these are both inserts they were gel extracted and purified. PCR purification is not carried out for inserts since they are small and would therefore be lost during the process.
Wednesday, August 18
- Replica plating and colony PCR of 5' ins and dif in pSB1C3 (bench ligation)
- Ligation of pveg (ES) and SpecR-T (XP) with pSB1C3 (EP)
pveg and SpecR-T (the front inserts) were ligated overnight with pSB1C3 (the vector).
Thursday, August 19
- Transformation of E.Coli with the overnights ligates
- 5' ins and dif in pSB1C3
- pveg and SpecR-T in pSB1C3
Friday, August 20
- Replica plating and colony PCR of both transformations from yesterday.
- Annealing the forward and reverse strands of the dif P ES oligo
The forward and reverse strands of the 3' dif Pme1 sites with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight.
Week 8
| Week 8 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
| Morning |
|
|
|
|
|
|
| Afternoon |
|
|
|
|
|
|
Week 9
| Week 9 | Monday | Tuesday | Wednesday | Thursday | Friday |
| Morning |
|
|
|
|
|
Afternoon |
|
|
|
|
|
Week 10
| Week 10 | Monday | Tuesday | Wednesday | Thursday | Friday |
| Morning |
|
|
|
|
|
Afternoon |
|
|
|
|
|
Week 11
| Week 11 | Monday | Tuesday | Wednesday | Thursday | Friday |
| Morning |
|
|
|
|
|
Afternoon |
|
|
|
|
|
Week 12
| Week 12 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
| Morning |
|
|
|
|
|
|
Afternoon |
|
|
|
|
|
|
Week 13
| Week 13 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
| Morning |
|
|
|
|
|
|
Afternoon |
|
|
|
|
|
|
AmyE Vector
Assembly of the AmyE vector Assembly
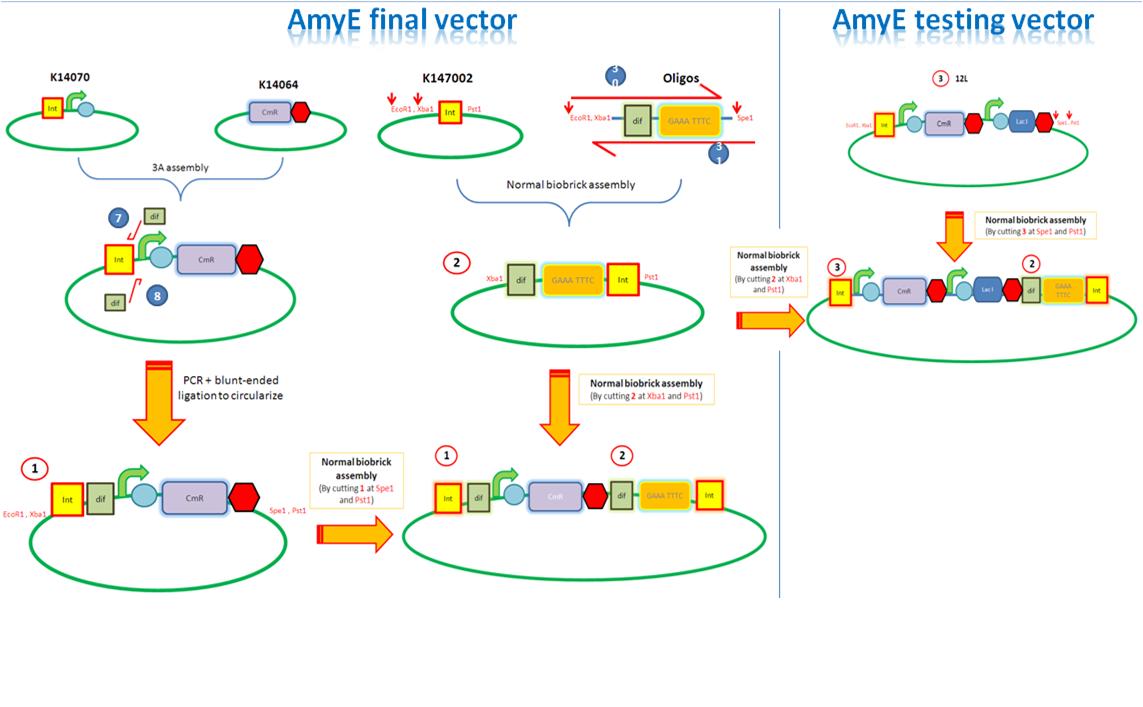
Starting from the top, we are assembling the first two fragments (K14070 and K14064) and K147002 with our oligos to add in a Dif site. Two dif sites on either side of resistance cassettes can be used to later excise antibiotic resistance from our final constructs.
K14070 and K14064 fragments
- DNA was taken out of the reigstry
- Cut with restriction enzymes
- Run on a gel to confirm correct cutting and estimate relative ratios of DNA for ligation
- Ligated overnight
- Transformed into E.Coli to Amplify the DNA
- Colony PCR has been used to confirm the correct insert size.
Next Steps:
- Extract the DNA with a miniprep
- Proceed to the next step - Reverse PCR
K14002 and oligos
- Ligated two single stranded oligos together to produce Dif and insertion site
- Standard biobrick assembly of oligos to K14002
- Ligation and transformation into E.coli competent cells (strain)
- Screen plated colonies for correct insertion
Next Steps:
- Purify the correct insert out of E.coli
- Next step assembly - LacI test vector and Final assembly vector
LacI Testing vector
Currently waiting for Part B step 2 Midi-prep results to start this step.
Schedule & Lab notes
Week 7
| Week 6 | Monday | Tuesday | Wednesday | Thursday | Friday |
|---|---|---|---|---|---|
| MORNING | Starting assembly of AmyE vector
|
|
|
k64 and k70, k70 only, Spec and 5' PyrD diff |
Spec and 5' PyrD diff
k64 and k70. k70 only was discarded since this was purely for a baground check |
| AFTERNOON |
|
|
|
|
Week 8
| Week 8 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|---|---|---|---|---|---|
| MORNING |
Set up overnight ligations for standard assembly (BBA) and 3A cloning (3A) of dif P
|
|
|
|
|
|
| AFTERNOON |
|
|
|
|
Backbone PCR of pSB1C3 using PFU (3rd attempt)
|
|
Week 9
| Week 8 | Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|---|
| MORNING |
|
|
|
|
| |
| AFTERNOON |
|
|
|
|
|
Week 10
| Week 8 | Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|---|
| MORNING |
|
|
|
|
| |
| AFTERNOON |
|
|
|
|
|
Week 11
| Week 8 | Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|---|
| MORNING |
|
|
|
|
| |
| AFTERNOON |
|
|
|
|
|
Week 12
| Week 8 | Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|---|
| MORNING |
|
|
|
|
| |
| AFTERNOON |
|
|
|
|
|
Week 13
| Week 8 | Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|---|
| MORNING |
|
|
|
|
| |
| AFTERNOON |
|
|
|
|
|
 "
"